Open Access Article
Open Access ArticleDiscrimination of cryptochirality in chiral isotactic polystyrene by asymmetric autocatalysis†
Tsuneomi Kawasakiab, Christiane Hohbergerc, Yuko Arakia, Kunihiko Hatasea, Klaus Beckerlec, Jun Okuda*c and Kenso Soai*ab
aDepartment of Applied Chemistry, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan. E-mail: soai@rs.kagu.tus.ac.jp; Fax: +81 3 5261 4631; Tel: +81 3 5228 8261
bResearch Institute of Science and Technology, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan
cInstitute of Inorganic Chemistry, RWTH Aachen University, Landoltweg 1, D-52074 Aachen, Germany. E-mail: jun.okuda@ac.rwth-aachen.de
First published on 21st August 2009
Abstract
Chiral isotactic polystyrenes induce the enantioselective addition of diisopropylzinc to pyrimidine-5-carbaldehyde, affording the enantiomerically enriched pyrimidyl alkanol with the corresponding absolute configuration to that of cryptochiral polystyrenes in conjunction with asymmetric autocatalysis.
Chirality plays a significant role in chemical, biological, pharmaceutical and material science.1 The study of chirality has been developed as an innovative technique2 for distinguishing between a pair of enantiomers. In general, optically active materials are chiral, but not all chiral materials are optically active. The optically inactive but chiral molecules exhibit cryptochirality.3,4
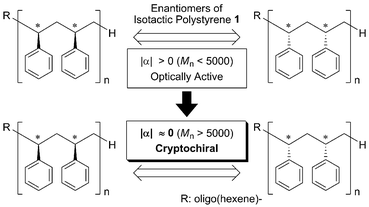
The phenomenon of cryptochirality can be observed in isotactic poly(α-olefins) such as polypropylenes and polystyrenes. Neglecting the chain ends, poly(α-olefins) feature a Cs symmetry and therefore possess a pseudo-mirror plane (Fig. 1).5 Because of the recent development of single-site catalysts for the synthesis of stereoregular and enantiomerically pure poly(α-olefins), it is now possible to correlate the chiroptical properties isotactic poly(α-olefins) with their chain length.6
 | ||
| Fig. 1 Cryptochirality in isotactic polystyrene 1. | ||
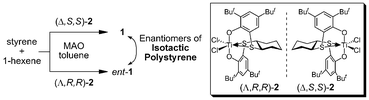
Previously we reported on the synthesis of enantioenriched isotactic polystyrene 1.7 It was prepared using a chiral titanium catalyst based on the (OSSO)-type ligand 27a,8 and 1-hexene as a chain transfer agent (Fig. 2). The dependence of the specific rotation value on the molecular weight of the isotactic polystyrenes has been demonstrated.7 Although low-molecular-weight polystyrene can be optically active, no optical activity was detected for high-molecular-weight polymers (Mn > 5000); i.e., high-molecular-weight polystyrene 1 possesses cryptochirality (Fig. 1).
 | ||
| Fig. 2 Synthesis of polystyrene 1 and the structure of the (OSSO)-type chiral ligand 2. | ||
How can one discriminate between the enantiomers of cryptochiral compounds? To the best of our knowledge, there is no contemporary method available to determine the cryptochirality of high-molecular-weight isotactic polystyrene. It should be noted that polystyrene does not have heteroatoms. Thus, the development of a method for the recognition of the enantiomeric forms of isotactic polystyrene is a challenge.
We developed a highly sensitive method to detect molecular chirality by using asymmetric autocatalysis9–12 with amplification of enantiomeric excess (ee).13 In the presence of a chiral compound, the enantioselective addition of diisopropylzinc (iPr2Zn) to a pyrimidine-5-carbaldehyde 3 affords highly enantioenriched (S)- or (R)-pyrimidyl alkanol 4, whose absolute configuration is efficiently controlled by the absolute configuration of an added chiral compound.14 Many types of chiral compounds have been used as chiral inducers in asymmetric autocatalysis, including cryptochiral small hydrocarbon molecules,15 but to date, there has been no report on the use of cryptochiral polymers that consist exclusively of hydrocarbons.
Here, we report on the highly enantioselective addition of iPr2Zn to pyrimidine-5-carbaldehyde 3 in the presence of enantioenriched isotactic polystyrene 1 and ent-1 (Mn 6000–6100 g mol−1) with no detectable optical rotation (Scheme 1). It was found that the cryptochirality of isotactic polystyrene was successfully recognized in conjunction with asymmetric autocatalysis.
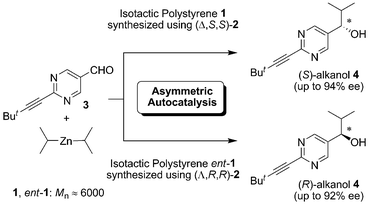 | ||
| Scheme 1 Enantioselective addition of iPr2Zn to aldehyde 3 induced by homochiral polystyrene 1, followed by asymmetric autocatalytic amplification of enantiomeric purity. | ||
Table 1 shows the results of the asymmetric autocatalysis triggered by enantiomers of the cryptochiral isotactic polystyrene 1. The stereochemical correlations of the cryptochirality and the absolute configurations of the resulting 5-pyrimidyl alkanol 4 are depicted. When iPr2Zn addition to pyrimidine-5-carbaldehyde 3 was performed in the presence of polystyrene 1, synthesized using (Δ,S,S)-2 as a polymerization catalyst, pyrimidyl alkanol 4 with S-configuration was obtained with 15% ee and 85% isolated yield (Table 1, entry 1). On the other hand, the enantiomer of isotactic polystyrene 1 (ent-1), prepared using (Λ,R,R)-2 as chiral ligand, afforded (R)-alkanol 4 with 5% ee in 89% isolated yield. The reproducibility of the stereochemical outcome is clearly shown in entries 3–8.
| Entrya | Optically inactive isotactic polystyrene 1b | Catalyst used for synthesis | 5-Pyrimidyl alkanol 4 | ||
|---|---|---|---|---|---|
| Isolated yield (%) | Ee (%)c | Config. | |||
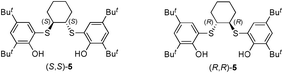
| a The molar ratio of polystyrene 1 : pyrimidine-5-carbaldehyde 3 : iPr2Zn = 0.018 : 0.525 : 1.18 (mmol). The general procedure for asymmetric autocatalysis (Table 1, entry 1) is as follows: iPr2Zn (0.08 mmol, 0.8 mL; 1.0 M toluene solution) was added dropwise to a toluene (0.75 mL) solution of polystyrene 1 (10 mg, ca. 0.018 mmol). To this solution was added a toluene (0.25 mL) solution of aldehyde 3 (4.7 mg, 0.025 mmol) over a period of 1.5 h at 0 °C. After stirring the mixture for 15 h, toluene (1 mL) and iPr2Zn (0.3 mmol, 0.3 mL; 1.0 M toluene solution) were then added at 0 °C, and the mixture was stirred for 1 h. A toluene (0.75 mL) solution of 3 (18.8 mg, 0.1 mmol) was slowly added, and the reaction mixture was stirred at 0 °C for 1.5 h. Then, toluene (5.0 mL), iPr2Zn (0.8 mmol, 0.8 mL; 1.0 M toluene solution) and a toluene (2.0 mL) solution of 3 (75.3 mg, 0.4 mmol) were added successively at 0 °C. After stirring the mixture for 2.5 h, the reaction was quenched with HCl (1 M, 3 mL) and neutralized with a saturated NaHCO3 solution (9 mL). The mixture was then filtered through Celite and the filtrate was extracted with AcOEt (three times). The combined organic layers were dried over anhydrous Na2SO4 and concentrated in vacuo. Purification of the residue by silica gel column chromatography on silica gel (hexane–AcOEt, 3 : 1, v/v) afforded the (S)-pyrimidyl alkanol 4 (103.1 mg, 0.444 mmol) in 85% yield. The ee value was determined to be 15% by HPLC, using a chiral stationary phase (Daicel Chiralpak IB 4.6Φ× 250 mm, 254 nm UV detector, room temperature, eluent: 5% 2-propanol in hexane (v/v), 1.0 mL min−1, retention time: 11 min for (S)-4 and 16 min for (R)-4).b For the synthesis and properties of 1, see ref. 7. Mn values are 6108 g mol−1 for 1 and 6006 g mol−1 for ent-1.c The ee value was determined by HPLC using a chiral stationary phase.d (R,R)-Bis(phenol) 5, which is a chiral organic part of catalyst (Λ,R,R)-2 was mixed with the polystyrene 1, and (R,R)-5 and polystyrene 1 were separated again using silica gel column chromatography before use as a chiral initiator of asymmetric autocatalysis. See also ref. 16.e (S,S)-Bis(phenol) 5 was mixed with the polystyrene ent-1, and (S,S)-5 and ent-1 are separated again using silica gel column chromatography before use as a chiral initiator of asymmetric autocatalysis. See also ref. 16. | |||||
| 1 | 1 | (Δ,S,S)-2 | 85 | 15 | S |
| 2 | ent-1 | (Λ,R,R)-2 | 89 | 5 | R |
| 3 | 1 | (Δ,S,S)-2 | 82 | 6 | S |
| 4 | ent-1 | (Λ,R,R)-2 | 88 | 4 | R |
| 5 | 1 | (Δ,S,S)-2 | 83 | 1.4 | S |
| 6 | ent-1 | (Λ,R,R)-2 | 83 | 1.1 | R |
| 7 | 1 | (Δ,S,S)-2 | 89 | 3 | S |
| 8 | ent-1 | (Λ,R,R)-2 | 84 | 3 | R |
| 9d | 1 | (Δ,S,S)-2 | 91 | 1.3 | S |
| 10e | ent-1 | (Λ,R,R)-2 | 88 | 3 | R |
The chiral catalyst 2 used in the polymerization reaction can be separated from the polystyrene 1 by purification using silica gel and a reprecipitation technique.7a Analytical thin layer chromatography was used to check for the absence of bis(phenol) 5, which is a chiral organic moiety of titanium complex 2, from polystyrene 1. However, considering the possibility of incomplete separation of chiral bis(phenol) 5 from cryptochiral polystyrene 1, and the asymmetric induction by the remaining bis(phenol) 5,16 isotactic polystyrene 1 used in entries 9 and 10 were prepared by mixing the opposite enantiomer of 5 to that utilized in the polymerization reaction, followed by repurification using silica gel column chromatography. The results of entries 9 and 10 strongly support the stereochemical correlations between cryptochiral 1/ent-1 and (S)/(R)-pyrimidyl alkanol 4. Although the enantiomeric excesses of the resulting pyrimidyl alkanol 4 reported in Table 1 are only in the range of 1.1–15% ee, it is possible to increase the ee value of the final product 4 by applying consecutive asymmetric autocatalysis.
In Table 2, entry 1, consecutive asymmetric autocatalysis13 was performed for the purpose of enhancing enantiomer purity to afford (S)-4 with 87% ee. Further asymmetric autocatalysis with amplification of ee has also been applied in entries 2–8 to afford highly enantioenriched (R) and (S)-alkanols 4 with 67–86% ee. It should be noted that the enantioselectivity in entries 1–8 was the same as observed in Table 1; i.e., isotactic polystyrene 1 obtained from the polymerization catalyzed by (Δ,S,S)-2 initiated the formation of (S)-alkanol 4, and ent-1 synthesized via catalysis of (Λ,R,R)-2 triggered the generation of alkanol 4 with R-configuration. Furthermore, an additional three rounds of asymmetric autocatalysis afforded the (S)- and (R)-product 4 with 94 and 92% ee, as shown in entries 9 and 10.
| Entrya | Optically inactive isotactic polystyrene 1b | Catalyst used for synthesis | 5-Pyrimidyl alkanol 4 | ||
|---|---|---|---|---|---|
| Isolated yield (%) | Ee (%)c | Config. | |||
| a On completion of the general experimental procedure, an additional two cycles of asymmetric autocatalysis with amplification of ee were performed. See ref. 13.b See Table 1, footnote b.c See Table 1, footnote c.d On completion of the general experimental procedure, an additional three cycles of asymmetric autocatalytic amplification were performed. See ref. 13. | |||||
| 1 | 1 | (Δ,S,S)-2 | 93 | 87 | S |
| 2 | ent-1 | (Λ,R,R)-2 | 85 | 77 | R |
| 3 | 1 | (Δ,S,S)-2 | 88 | 67 | S |
| 4 | ent-1 | (Λ,R,R)-2 | 87 | 84 | R |
| 5 | 1 | (Δ,S,S)-2 | 93 | 78 | S |
| 6 | ent-1 | (Λ,R,R)-2 | 93 | 63 | R |
| 7 | 1 | (Δ,S,S)-2 | 94 | 86 | S |
| 8 | ent-1 | (Λ,R,R)-2 | 91 | 69 | R |
| 9d | 1 | (Δ,S,S)-2 | 99 | 94 | S |
| 10d | ent-1 | (Λ,R,R)-2 | 99 | 92 | R |
In this enantioselective reaction, cryptochirality caused by a small relative difference between the end groups of the enantiomeric polystyrene chains may control the si- or re-enantioface selection of iPr2Zn addition to pyrimidine-5-carbaldehyde 3 to afford the isopropylzinc alkoxide of pyrimidyl alkanol 4 (autocatalyst) with a minute enantiomeric imbalance. Once the asymmetric autocatalyst with a small enantiomeric enrichment is generated, the enantiomeric purity of pyrimidyl alkanol 4 increases during the addition of iPr2Zn to aldehyde 3 thanks to the asymmetric autocatalysis.17 Thus, chiral alkanol 4 with an absolute configuration corresponding to that of chiral polymer 1 is formed in a highly enantioenriched form. We can therefore distinguish the enantiomeric form of cryptochiral polystyrene 1 by determining the absolute configuration of the resulting alkanol 4. The detailed mechanism of the amplification of enantiomeric excess in asymmetric autocatalysis is now under investigation. The results will be reported in due course.
On the other hand, when polystyrenes with higher molecular weights (Mn > ca. 600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) were used in the asymmetric autocatalysis, no reproducible results were obtained (thus far). This may be ascribed to further minute cryptochirality of 1, the occurrence of stereoerrors and/or low solubility in toluene.
000 g mol−1) were used in the asymmetric autocatalysis, no reproducible results were obtained (thus far). This may be ascribed to further minute cryptochirality of 1, the occurrence of stereoerrors and/or low solubility in toluene.
In summary, we have demonstrated that asymmetric autocatalysis is a useful method for sensing cryptochirality of homochiral isotactic polystyrene 1. Homochiral polystyrenes autocatalytically induce the enantioselective addition of iPr2Zn to pyrimidine-5-carbaldehyde 3 to afford the pyrimidyl alkanol 4 with the corresponding absolute configurations to that of cryptochiral polystyrenes. Cryptochirality of isotactic polystyrene, which cannot be distinguished between the enantiomeric forms by applying any contemporary technique, can be discriminated as the visible chirality of autocatalyst 4 by asymmetric autocatalysis with amplification of chirality.
Notes and references
- E. L. Eliel and S. W. Wilen, Stereochemistry of Organic Compounds, Wiley, New York, 1994 Search PubMed.
- (a) K. Nakanishi, N. Berova and R. W. Woody, Circular Dichroism: Principles and Applications, Wiley, New York, 2000 Search PubMed; (b) J. Haesler, I. Schindelholz, E. Riguet, C. G. Bochet and W. Hug, Nature, 2007, 446, 526 CrossRef CAS; (c) L. A. Nafie and T. B. Freedman, Enantiomer, 1998, 3, 283 CAS; (d) L. D. Barron, Nature, 1972, 238, 17 CrossRef CAS; (e) Y. Okamoto, E. Yashima and C. Yamamoto, Top. Stereochem., 2003, 24, 157 Search PubMed; (f) U. J. Meierhenrich, W. H.-P. Thiemann, F. Goesmann, R. Roll and H. Rosenbauer, Chirality, 2001, 13, 454 CrossRef CAS.
- (a) K. Mislow and P. Bickart, Isr. J. Chem., 1976–1977, 15, 1 Search PubMed; (b) K. Mislow, Collect. Czech. Chem. Commun., 2003, 68, 849 CrossRef CAS.
- (a) M. M. Green and S. K. Jha, Chirality, 1997, 9, 424 CrossRef CAS; (b) C. W. Thomas and Y. Tor, Chirality, 1998, 10, 53 CrossRef CAS.
- (a) M. Farina, Top. Stereochem., 1987, 17, 1 Search PubMed; (b) P. Pino, P. Cioni and J. Wei, J. Am. Chem. Soc., 1987, 109, 6189 CrossRef CAS; (c) K. Nozaki, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 215 CrossRef CAS.
- J.-F. Carpentier, Angew. Chem., Int. Ed., 2007, 46, 6404 CrossRef.
- (a) K. Beckerle, R. Manivannan, B. Lian, G.-J. M. Meppelder, G. Raabe, T. P. Spaniol, H. Ebeling, F. Pelascini, R. Mülhaupt and J. Okuda, Angew. Chem., Int. Ed., 2007, 46, 4790 CrossRef CAS; (b) G.-J. M. Meppelder, K. Beckerle, R. Manivannan, B. Lian, G. Raabe, T. P. Spaniol and J. Okuda, Chem.–Asian J., 2008, 3, 1312 CrossRef CAS.
- Experimental procedure for a related racemic ligand, see: K. Marcseková, C. Loos, F. Rominger and S. Doye, Synlett, 2007, 16, 2564 Search PubMed.
- K. Soai, T. Shibata, H. Morioka and K. Choji, Nature, 1995, 378, 767 CrossRef CAS.
- Reviews by other groups: (a) J. Podlech and T. Gehring, Angew. Chem., Int. Ed., 2005, 44, 5776 CrossRef CAS; (b) C. Bolm, F. Bienewald and A. Seger, Angew. Chem., Int. Ed. Engl., 1996, 35, 1657 CrossRef CAS; (c) M. H. Todd, Chem. Soc. Rev., 2002, 31, 211 RSC; (d) M. Avalos, R. Babiano, P. Cintas, J. L. Jiménez and J. C. Palacios, Chem. Commun., 2000, 887 RSC; (e) H. Buschmann, R. Thede and D. Heller, Angew. Chem., Int. Ed., 2000, 39, 4033 CrossRef CAS; (f) K. Mikami and M. Yamanaka, Chem. Rev., 2003, 103, 3369 CrossRef CAS; (g) I. D. Gridnev, Chem. Lett., 2006, 35, 148 CrossRef CAS; (h) L. Caglioti, C. Zucchi and G. Pályi, Chemistry Today (Chimica Oggi), 2005, 23, 38 Search PubMed.
- Reviews by our group: (a) K. Soai, T. Shibata and I. Sato, Acc. Chem. Res., 2000, 33, 382 CrossRef CAS; (b) K. Soai, T. Shibata and I. Sato, Bull. Chem. Soc. Jpn., 2004, 77, 1063 CrossRef CAS; (c) K. Soai and T. Kawasaki, Chirality, 2006, 18, 469 CrossRef CAS; (d) K. Soai and T. Kawasaki, Top. Curr. Chem., 2008, 284, 1 CAS; (e) K. Soai and T. Kawasaki, in Organometallic Chirality, ed. G. Palyi, C. Zucchi and L. Caglioti, Mucchi Editore, Modena, 2008, ch. 6, p. 107 Search PubMed.
- (a) T. Shibata, H. Morioka, T. Hayase, K. Choji and K. Soai, J. Am. Chem. Soc., 1996, 118, 471 CrossRef CAS; (b) T. Shibata, S. Yonekubo and K. Soai, Angew. Chem., Int. Ed., 1999, 38, 659 CrossRef CAS; (c) I. Sato, T. Yanagi and K. Soai, Chirality, 2002, 14, 166 CrossRef CAS; (d) F. Lutz, T. Kawasaki and K. Soai, Tetrahedron: Asymmetry, 2006, 17, 486 CrossRef CAS.
- I. Sato, H. Urabe, S. Ishiguro, T. Shibata and K. Soai, Angew. Chem., Int. Ed., 2003, 42, 315 CrossRef CAS.
- (a) T. Shibata, J. Yamamoto, N. Matsumoto, S. Yonekubo, S. Osanai and K. Soai, J. Am. Chem. Soc., 1998, 120, 12157 CrossRef CAS; (b) K. Soai, S. Osanai, K. Kadowaki, S. Yonekubo, T. Shibata and I. Sato, J. Am. Chem. Soc., 1999, 121, 11235 CrossRef CAS; (c) I. Sato, D. Omiya, T. Saito and K. Soai, J. Am. Chem. Soc., 2000, 122, 11739 CrossRef CAS; (d) T. Kawasaki, M. Sato, S. Ishiguro, T. Saito, Y. Morishita, I. Sato, H. Nishino, Y. Inoue and K. Soai, J. Am. Chem. Soc., 2005, 127, 3274 CrossRef CAS; (e) T. Kawasaki, K. Suzuki, Y. Hakoda and K. Soai, Angew. Chem., Int. Ed., 2008, 47, 496 CrossRef CAS; (f) T. Kawasaki, Y. Matsumura, T. Tsutsumi, K. Suzuki, M. Ito and K. Soai, Science, 2009, 324, 492 CrossRef CAS.
- T. Kawasaki, H. Tanaka, T. Tsutsumi, T. Kasahara, I. Sato and K. Soai, J. Am. Chem. Soc., 2006, 128, 6032 CrossRef CAS.
- The direction of asymmetric induction has been examined employing (S,S) and (R,R)-bis(phenol) 5 as a chiral initiator of asymmetric autocatalysis, respectively. As a result of asymmetric autocatalysis, (S,S)-bis(phenol) 5 induced the formation of (S)-pyrimidyl alkanol 4 with a high ee value, and (R,R)-5 promoted the production of enantiomerically enriched (R)-alkanol 4, respectively. The results are detailed in the ESI†.
. - (a) I. Sato, D. Omiya, H. Igarashi, K. Kato, Y. Ogi, K. Tsukiyama and K. Soai, Tetrahedron: Asymmetry, 2003, 14, 975 CrossRef CAS; (b) I. D. Gridnev, J. M. Serafimov and J. M. Brown, Angew. Chem., Int. Ed., 2004, 43, 4884 CrossRef CAS; (c) D. G. Blackmond, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 5732 CrossRef CAS; (d) J. R. Islas, D. Lavabre, J.-M. Grevy, R. H. Lamoneda, H. R. Cabrera, J.-C. Micheau and T. Buhse, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 13743 CrossRef CAS; (e) Y. Saito and H. Hyuga, Top. Curr. Chem., 2008, 284, 97 CAS; (f) G. Lente, J. Phys. Chem. A, 2005, 109, 11058 CrossRef CAS; (g) K. Micskei, G. Pota, L. Caglioti and G. Palyi, J. Phys. Chem. A, 2006, 110, 5982 CrossRef CAS; (h) J. M. Brown, I. Gridnev and J. Klankermayer, Top. Curr. Chem., 2008, 284, 35 CAS; (i) D. Lavabre, J.-C. Micheau, J. R. Islas and T. Buhse, Top. Curr. Chem., 2008, 284, 67 CAS; (j) F. Lutz, T. Igarashi, T. Kinoshita, M. Asahina, K. Tsukiyama, T. Kawasaki and K. Soai, J. Am. Chem. Soc., 2008, 130, 2956 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The asymmetric autocatalysis induced by bis(phenol) 5. See DOI: 10.1039/b912813a |
| This journal is © The Royal Society of Chemistry 2009 |