 Open Access Article
Open Access ArticleAsymmetric autocatalysis induced by meteoritic amino acids with hydrogen isotope chirality†‡
Tsuneomi
Kawasaki
ab,
Masako
Shimizu
a,
Daisuke
Nishiyama
a,
Masateru
Ito
a,
Hitomi
Ozawa
a and
Kenso
Soai
*ab
aDepartment of Applied Chemistry, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan. E-mail: soai@rs.kagu.tus.ac.jp; Fax: +81 3 5261 4631; Tel: +81 3 5228 8261
bResearch Institute of Science and Technology, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan
First published on 24th June 2009
Abstract
Achiral meteoritic amino acids, glycine and α-methylalanine, with hydrogen isotope (D/H) chirality, acted as the source of chirality in asymmetric autocatalysis with amplification of ee to afford highly enantioenriched 5-pyrimidyl alkanols.
One of the most fascinating subjects about the prebiotic world is the origin of homochirality, as in L-amino acids and D-sugars.1 According to the theory of the extraterrestrial origin of biological homochirality, the amino acids in meteorites have been considered to play an important role in molecular evolution2 because a variety of prebiotic organic molecules, especially L-enriched amino acids with only slight enantiomeric excess (ee), have been identified.3 Their stable-isotope enrichment (2H, 13C and 15N) supports an extraterrestrial origin for these chiral amino acids.4
On the other hand, meteorites also include achiral amino acids such as glycine and α-methylalanine that have also been identified as isotopically enriched relative to terrestrial compounds.4c The δD value of Vienna standard mean ocean water of meteoritic glycine and α-methylalanine appeared to be 399‰ and 3097‰, in contrast to the terrestrial biogenic compounds, which range from 350‰ to 50‰. Although glycine and α-methylalanine composed of 1H are achiral, this is not the case of meteoritic compounds because their hydrogen isotope ratio is high and should include the isotopically chiral components. Deuteration of the methylene group of glycine and one methyl group of α-methylalanine produces the hydrogen isotopically chiral compounds glycine-α-d1 and α-methyl-d3-alanine 2 (Fig. 1).
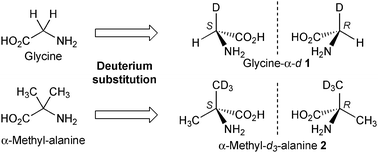 | ||
| Fig. 1 Generation of chirality by the deuterium substitution of enantiotopic hydrogen in glycine and α-methylalanine. | ||
To the best of our knowledge, there are no reports on the analysis of isotopic chirality of achiral meteoritic amino acids. Analysis of the isotopic chirality in meteoritic achiral molecules is an important subject to help understand the possible role of these compounds as the origin of chirality. In addition, it may expand research on the pathway by which chirality and isotope enrichment are produced in the meteoritic compounds in the universe.
Although there are a few reports on asymmetric reactions,1g,5 and separation6 induced by hydrogen isotope chirality, unlike usual chiral compounds the recognition of the enantiomers solely as a result of isotope labeling still remains difficult. Thus, the development of a highly sensitive method for the detection of isotopic chirality in meteoritic organic compounds, especially amino acids, which are identified in carbonaceous chondrites4c with an achiral framework and isotopic enrichment, is a challenging subject.
We and others have extensively studied asymmetric autocatalysis with amplification of ee.7–11 We have also reported that the external chiral initiator,12 such as chiral natural amino acids,12e in the reaction of pyrimidine-5-carbaldehyde and diisopropylzinc (i-Pr2Zn) controls the absolute configuration of the produced 5-pyrimidyl alkanols and that the ee of the produced pyrimidyl alkanols is high, in conjunction with asymmetric autocatalysis. Chiral deuterated primary alcohols and carbon isotopically chiral compounds can work as chiral triggers of this autocatalytic amplification of ee.13
We report here that the isotopically chiral glycine-α-d (1)14 and α-methyl-d3-alanine (2)15 induce the enantioselective addition of i-Pr2Zn to pyrimidine-5-carbaldehyde 3 to afford, in combination with asymmetric autocatalysis, pyrimidyl alkanol 4 with significantly high ee. The absolute configuration of the corresponding alkanol 4 was controlled efficiently by the chirality resulting from hydrogen isotope substitution of 1 and 2 (Scheme 1). Hydrogen isotope enantiomers of compounds 1 and 2 were synthesized by a previously reported method14,15 and the ee was determined from the 1H NMR spectrum of the camphanyl amide of 1 and the α-methoxy-α-(trifluoromethyl)phenylacetamide (MTPA amide) of 2 by the diastereomer method.
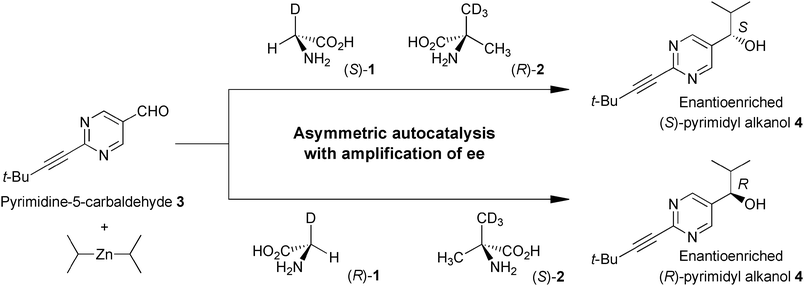 | ||
| Scheme 1 Isotopically chiral amino acid induced asymmetric autocatalysis of 5-pyrimidyl alkanol 4 in the addition of i-Pr2Zn to aldehyde 3. | ||
The results of asymmetric autocatalysis triggered by isotopically chiral amino acids are summarized in Table 1. When the i-Pr2Zn addition was performed in the presence of the (S)-glycine-α-d (1) with an ee of 93%, the (S)-5-pyrimidyl alkanol 4 was obtained in a 94% yield with an ee of 96% (Table 1, series I, entry 1). The results were reproducible. Thus, (S)-4 with an ee of 95% was obtained in the presence of (S)-1 (entry 2). The asymmetric autocatalysis using (S)-1 with 94% ee prepared in a different batch supported the reproducibility; that is, (S)-1 induces the formation of (S)-4 with high ee (entries 3, 4). On the other hand, in the presence of the (R)-1 instead of the (S)-1 enantiomer, the reaction between aldehyde 3 and i-Pr2Zn always gave (R)-4 with an ee of 91–93% in a yield of 90–94% (entries 5–8). These results clearly exhibit the correlation between the chirality of the glycine-α-d (1) and the absolute configuration of the resulting alkanol 4.
| Pyrimidyl alkanol 4 | ||||
|---|---|---|---|---|
| Entry | Amino acid a (% ee) | Yieldb (%) | eec (%) | Config. |
| a The ee of 1 and 2 were determined from the 1H NMR spectrum of the camphanyl amide of 1 and the MTPA amide of 2. b Isolated yield. c The ee was determined using HPLC employing a chiral stationary phase. d The molar ratio was 1/3/i-Pr2Zn = 0.05/1.05/2.2. The aldehyde 3 and i-Pr2Zn were added in three separate portions. Experimental details are as follows (entry 1): i-Pr2Zn (0.15 mL of 1 M methylcyclohexane (MCH) solution, 0.15 mmol) was added to (S)-1 (3.8 mg, 0.05 mmol) and the mixture was stirred for 12 h at room temperature. After the addition of i-Pr2Zn (0.5 mL of 1 M MCH solution, 0.5 mmol,) at 0 °C, a MCH (2.0 mL) solution of 3 (9.4 mg, 0.05 mmol) was added over a period of 1 h and the mixture was stirred for 6 h at 0 °C. After toluene (3.2 mL) and i-Pr2Zn (0.4 mL of 1 M toluene solution, 0.4 mmol) were added, the reaction mixture was stirred for 10 min. Then, a toluene (1.5 mL) solution of 3 (37.6 mg, 0.2 mmol) was added over a period of 1 h at 0 °C, and the mixture was stirred for 2 h. Moreover, after toluene (14.4 mL) and i-Pr2Zn (1.6 mL of 1 M toluene solution) were added and the mixture was stirred for 10 min, a toluene (4.0 mL) solution of 3 (150.6 mg, 0.8 mmol) was added over a period of 1 h. After stirring for 1 h, the reaction was quenched with 1 M aqueous hydrochloric acid (5 mL) at 0 °C. After neutralization with saturated aqueous sodium hydrogen carbonate (15 mL), the mixture was filtered through Celite, and the filtrate was extracted with ethyl acetate. The combined organic fractions were dried over anhydrous sodium sulfate and evaporated in vacuo. Purification of the residue using silica gel thin layer chromatography (hexane–ethyl acetate = 2/1, v/v) gave (S)-4 (229 mg, 0.9849 mmol, 96% ee) in 94% yield. e The molar ratio was 2/3/i-Pr2Zn = 0.075/0.625/1.35. The aldehyde 3 and i-Pr2Zn were added in four separate portions. f Each pair of reactions (9/13 and 10/14) was performed using the same apparatus to exclude any effect other than that of isotopically chiral amino acid 2. | ||||
| Series I (glycine-α-d1)d | ||||
| 1 | (S)-1 (93) | 94 | 96 | S |
| 2 | (S)-1 (93) | 98 | 95 | S |
| 3 | (S)-1 (94) | 94 | 94 | S |
| 4 | (S)-1 (94) | 95 | 93 | S |
| 5 | (R)-1 (96) | 94 | 93 | R |
| 6 | (R)-1 (96) | 94 | 91 | R |
| 7 | (R)-1 (92) | 90 | 91 | R |
| 8 | (R)-1 (92) | 93 | 92 | R |
| Series II (α-methyl-d3-alanine 2)e | ||||
| 9f | (R)-2 (81) | 95 | 99 | S |
| 10f | (R)-2 (81) | 97 | 98 | S |
| 11 | (R)-2 (81) | 94 | 96 | S |
| 12 | (R)-2 (81) | 92 | 97 | S |
| 13f | (S)-2 (69) | 99 | 98 | R |
| 14f | (S)-2 (69) | 93 | 99 | R |
| 15 | (S)-2 (69) | 84 | 97 | R |
| 16 | (S)-2 (69) | 93 | 96 | R |
Next, we examined the highly enantioselective addition of i-Pr2Zn to pyrimidine-5-carbaldehyde 3 using the (R)- and (S)-α-methyl-d3-alanine (2). In the presence of (R)-α-methyl-d3-alanine (2), (S)-5-pyrimidyl alkanol 4 was induced, and was obtained with an ee of 96–99% (series II, entries 9–12). On the other hand, in the presence of (S)-2, the reaction between aldehyde 3 and i-Pr2Zn always gave (R)-alkanol 4 with an ee of 96–99% in a yield of 84–99% (entries 13–16). To exclude any effect other than that of the α-methyl-d3-alanine (2), both reactions (entries 9, 13 and 10, 14) induced by (R)- and (S)-2 were performed using the same apparatus to give the (S)- and (R)-alkanols 4, respectively.
The chirality of these enantiomers is mainly due to the very small difference between the lengths of the C–D and C–H bonds.16 The high ee observed in the above asymmetric reactions may be explained as follows. In the initial step of the reaction, the isotopically chiral amino acid (or its zinc salt) induces a slight enantiomeric imbalance in the addition of i-Pr2Zn to 3. The resulting isopropylzinc alkoxide of 4 has a small ee, which possesses the absolute configuration corresponding to that of the isotopically chiral amino acid. Then, the ee is enhanced during the subsequent asymmetric autocatalysis followed by hydrolysis, which affords alkanol 4 with high ee.13
In conclusion, the enantioselective addition of i-Pr2Zn to pyrimidine-5-carbaldehyde 3 was achieved by utilizing the hydrogen isotopic chirality of achiral amino acids, that is, glycine-α-d (1) and α-methyl-d3-alanine (2). The observed ee of the produced pyrimidyl alkanol 4 was amplified up to 99% ee in conjunction with asymmetric autocatalysis. This is the first example of a highly enantioselective reaction induced by the chirality resulting from deuterium substitution of amino acids, which are detected in meteorites as achiral isotopically enriched molecules. We believe that the asymmetric autocatalysis significantly increases the value of the implications of isotope substitution in achiral meteoritic amino acids in the study of the origin and prebiotic evolution of biological homochirality.
Notes and references
- (a) K. Mislow, Collect. Czech. Chem. Commun., 2003, 68, 849–864 CrossRef CAS; (b) B. L. Feringa and R. A. van Delden, Angew. Chem., Int. Ed., 1999, 38, 3418–3438 CrossRef; (c) M. Bolli, R. Micula and A. Eschenmoser, Chem. Biol., 1997, 4, 309–320 CrossRef CAS; (d) W. A. Bonner, Origins Life Evol. Biosphere, 1995, 25, 175–190 CrossRef CAS; (e) C. Girard and H. B. Kagan, Angew. Chem., Int. Ed., 1998, 37, 2923–2959 CrossRef CAS; (f) H. Zepik, E. Shavit, M. Tang, T. R. Jensen, K. Kjaer, G. Bolbach, L. Leiserowitz, I. Weissbuch and M. Lahav, Science, 2002, 295, 1266–1269 CrossRef CAS; (g) M. M. Green, J.-W. Park, T. Sato, A. Teramoto, S. Lifson, R. L. B. Selinger and J. V. Selinger, Angew. Chem., Int. Ed., 1999, 38, 3139–3154 CAS; (h) J. M. Ribó, J. Crusats, F. Sagues, J. M. Claret and R. Rubires, Science, 2001, 292, 2063–2066 CrossRef CAS; (i) B. Barabás, L. Caglioti, K. Micskei, C. Zucchi and G. Pályi, Origins Life Evol. Biosphere, 2008, 38, 317–327 CrossRef CAS; (j) A. Guijarro and M. Yus, The Origin of Chirality in the Molecules of Life, The Royal Society of Chemistry, Cambridge, 2009 Search PubMed.
- (a) W. Notz and B. List, J. Am. Chem. Soc., 2000, 122, 7386–7387 CrossRef CAS; (b) S. Pizarello and A. L. Weber, Science, 2004, 303, 1151–1151 CrossRef CAS; (c) A. Córdova, M. Engqvist, I. Ibrahem, J. Casas and H. Sundén, Chem. Commun., 2005, 2047–2049 RSC.
- (a) K. Kvenvolden, Nature, 1970, 228, 923–926 CrossRef CAS; (b) J. R. Cronin and S. Pizzarello, Science, 1997, 275, 951–955 CrossRef CAS; (c) M. H. Engel and B. Nagy, Nature, 1982, 296, 837–840 CrossRef CAS; (d) D. O. Glavin and J. P. Dworkin, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 5487–5492 CrossRef CAS.
- (a) S. Epstein, R. V. Krishnamurthy, J. R. Cronin, S. Pizzarello and G. U. Yuen, Nature, 1987, 326, 477–479 CrossRef CAS; (b) M. H. Engel and S. A. Macko, Nature, 1997, 389, 265–268 CrossRef CAS; (c) S. Pizzarello and Y. Huang, Geochim. Cosmochim. Acta, 2005, 69, 599–605 CrossRef CAS.
- (a) H. Pracejus, Tetrahedron Lett., 1966, 7, 3809–3813 CrossRef; (b) A. Horeau, A. Nouaille and K. Mislow, J. Am. Chem. Soc., 1965, 87, 4957–4958 CrossRef.
- (a) K. Kimata, M. Kobayashi, K. Hosoya, T. Araki and N. Tanaka, J. Am. Chem. Soc., 1996, 118, 759–762 CrossRef CAS; (b) W. H. Pirkle and K. Z. Gan, Tetrahedron: Asymmetry, 1997, 8, 811–814 CrossRef CAS; (c) R. N. Harris III, P. Sundararaman and C. Djerassi, J. Am. Chem. Soc., 1983, 105, 2408–2413 CrossRef.
- K. Soai, T. Shibata, H. Morioka and K. Choji, Nature, 1995, 378, 767–768 CrossRef CAS.
- (a) I. Sato, H. Urabe, S. Ishiguro, T. Shibata and K. Soai, Angew. Chem., Int. Ed., 2003, 42, 315–317 CrossRef CAS; (b) T. Shibata, S. Yonekubo and K. Soai, Angew. Chem., Int. Ed., 1999, 38, 659–661 CrossRef CAS; (c) T. Shibata, H. Morioka, T. Hayase, K. Choji and K. Soai, J. Am. Chem. Soc., 1996, 118, 471–472 CrossRef CAS; (d) F. Lutz, T. Kawasaki and K. Soai, Tetrahedron: Asymmetry, 2006, 17, 486–490 CrossRef CAS.
- (a) J. Podlech and T. Gehring, Angew. Chem., Int. Ed., 2005, 44, 5776–5777 CrossRef CAS; (b) C. Bolm, F. Bienewald and A. Seger, Angew. Chem., Int. Ed. Engl., 1996, 35, 1657–1659 CrossRef CAS; (c) M. H. Todd, Chem. Soc. Rev., 2002, 31, 211–222 RSC; (d) M. Avalos, R. Babiano, P. Cintas, J. L. Jiménez and J. C. Palacios, Chem. Commun., 2000, 887–892 RSC; (e) H. Buschmann, R. Thede and D. Heller, Angew. Chem., Int. Ed., 2000, 39, 4033–4036 CrossRef CAS; (f) K. Mikami and M. Yamanaka, Chem. Rev., 2003, 103, 3369–3400 CrossRef CAS; (g) I. D. Gridnev, Chem. Lett., 2006, 35, 148–153 CrossRef CAS; (h) L. Caglioti, C. Zucchi and G. Pályi, Chim. Oggi, 2005, 23, 38–43 Search PubMed.
- (a) K. Soai, T. Shibata and I. Sato, Bull. Chem. Soc. Jpn., 2004, 77, 1063–1073 CrossRef CAS; (b) K. Soai, T. Shibata and I. Sato, Acc. Chem. Res., 2000, 33, 382–390 CrossRef CAS; (c) K. Soai and T. Kawasaki, Chirality, 2006, 18, 469–478 CrossRef CAS; (d) K. Soai and T. Kawasaki, Top. Curr. Chem., 2008, 284, 1–33 CAS; (e) K. Soai and T. Kawasaki, in Organometallic Chirality, ed. G. Palyi, C. Zucchi and L. Caglioti, Mucchi Editore, Modena, 2008, ch. 6, pp. 107–125 Search PubMed.
- (a) I. Sato, D. Omiya, H. Igarashi, K. Kato, Y. Ogi, K. Tsukiyama and K. Soai, Tetrahedron: Asymmetry, 2003, 14, 975–979 CrossRef CAS; (b) I. D. Gridnev, J. M. Serafimov and J. M. Brown, Angew. Chem., Int. Ed., 2004, 43, 4884–4887 CrossRef CAS; (c) D. G. Blackmond, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 5732–5736 CrossRef CAS; (d) J. R. Islas, D. Lavabre, J.-M. Grevy, R. H. Lamoneda, H. R. Cabrera, J.-C. Micheau and T. Buhse, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 13743–13748 CrossRef CAS; (e) Y. Saito and H. Hyuga, J. Phys. Soc. Jpn., 2004, 73, 33–35 CrossRef CAS; (f) G. Lente, J. Phys. Chem. A, 2005, 109, 11058–11063 CrossRef CAS; (g) K. Micskei, G. Pota, L. Caglioti and G. Palyi, J. Phys. Chem. A, 2006, 110, 5982–5984 CrossRef CAS; (h) J. Klankermayer, I. D. Gridnev and J. M. Brown, Chem. Commun., 2007, 3151 RSC; (i) F. Lutz, T. Igarashi, T. Kawasaki and K. Soai, J. Am. Chem. Soc., 2005, 127, 12206–12207 CrossRef CAS; (j) F. Lutz, T. Igarashi, T. Kinoshita, M. Asahina, K. Tsukiyama, T. Kawasaki and K. Soai, J. Am. Chem. Soc., 2008, 130, 2956–2958 CrossRef CAS; (k) J. M. Brown, I. Gridnev and J. Klankermayer, Top. Curr. Chem., 2008, 284, 35–65 CAS; (l) D. Lavabre, J.-C. Micheau, J. R. Islas and T. Buhse, Top. Curr. Chem., 2008, 284, 67–96 CAS; (m) Y. Saito and H. Hyuga, Top. Curr. Chem., 2008, 284, 97–118 CAS; (n) L. Schiaffino and G. Ercolani, Angew. Chem., Int. Ed., 2008, 47, 6832–6835 CrossRef CAS.
- (a) T. Shibata, J. Yamamoto, N. Matsumoto, S. Yonekubo, S. Osanai and K. Soai, J. Am. Chem. Soc., 1998, 120, 12157–12158 CrossRef CAS; (b) K. Soai, S. Osanai, K. Kadowaki, S. Yonekubo, T. Shibata and I. Sato, J. Am. Chem. Soc., 1999, 121, 11235–11236 CrossRef CAS; (c) T. Kawasaki, M. Sato, S. Ishiguro, T. Saito, Y. Morishita, I. Sato, H. Nishino, Y. Inoue and K. Soai, J. Am. Chem. Soc., 2005, 127, 3274–3235 CrossRef CAS; (d) T. Kawasaki, H. Tanaka, T. Tsutsumi, T. Kasahara, I. Sato and K. Soai, J. Am. Chem. Soc., 2006, 128, 6032–6033 CrossRef CAS; (e) I. Sato, Y. Ohgo, H. Igarashi, D. Nishiyama, T. Kawasaki and K. Soai, J. Organomet. Chem., 2007, 692, 1783–1787 CrossRef CAS; (f) T. Kawasaki, K. Suzuki, Y. Hakoda and K. Soai, Angew. Chem., Int. Ed., 2008, 47, 496–499 CrossRef CAS; (g) T. Kawasaki, T. Omine, K. Suzuki, H. Sato, A. Yamagishi and K. Soai, Org. Biomol. Chem., 2009, 7, 1073–1075 RSC.
- (a) I. Sato, D. Omiya, T. Saito and K. Soai, J. Am. Chem. Soc., 2000, 122, 11739–11740 CrossRef CAS; (b) T. Kawasaki, Y. Matsumura, T. Tsutsumi, K. Suzuki, M. Ito and K. Soai, Science, 2009, 324, 492–495 CrossRef CAS.
- (a) J. R. Walker and W. Curley, Jr., Tetrahedron, 2001, 57, 6695–6701 CrossRef CAS; (b) R. M. Williams, D. Zhai and Peter J. Sinclair, J. Org. Chem., 1986, 51, 5021–5022 CrossRef CAS.
- cf. D. Seebach and A. Fadel, Helv. Chim. Acta, 1985, 68, 1243–1250 Search PubMed.
- (a) L. S. Bartell, E. A. Roth, C. D. Hollowell, K. Kuchitsu and J. E. Young, Jr., J. Chem. Phys., 1965, 42, 2683–2686 CrossRef CAS; (b) L. S. Bartell and R. R. Roskos, J. Chem. Phys., 1966, 44, 457–463 CrossRef CAS.
Footnotes |
| † This article is part of a ChemComm ‘Catalysis in Organic Synthesis’ web-theme issue showcasing high quality research in organic chemistry. Please see our website (http://www.rsc.org/chemcomm/organicwebtheme2009) to access the other papers in this issue. |
| ‡ Electronic supplementary information (ESI) available: Procedures for the asymmetric autocatalysis in the presence of 1 and 2, the preparation of enantiomers of 1 and 2, and the determination of the enantiomeric purity of 1 and 2. See DOI: 10.1039/b908754k |
| This journal is © The Royal Society of Chemistry 2009 |
