Solubility adjustable nanoparticles stabilized by a novel PVP based family: synthesis, characterization and catalytic properties†
Ning
Yan
,
Jia-guang
Zhang
,
Yinyin
Tong
,
Siyu
Yao
,
Chaoxian
Xiao
,
Zichen
Li
and
Yuan
Kou
*
PKU Green Chemistry Centre, Beijing National Laboratory for Molecular Sciences, College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, China. E-mail: yuankou@pku.edu.cn; Fax: +86-10-62751708; Tel: +86-10-62757792
First published on 12th June 2009
Abstract
Systematic fabrication of nanoparticle stabilizers can substantially modify the properties of prepared nanoparticles; here the synthesis of solubility adjustable nanoparticles is achieved by employing a family of polarity modulated stabilizers.
Metal nanoparticles (MNPs), due to their promising optical, electronic, magnetic and catalytic properties, have received intense and continuous interest over the last few years.1 Recent advances have already led to the success in controlled synthesis of MNPs with different size, morphology, structure and composition.2 It is then highly desirable that the resulting MNPs can be dissolved in a given solvent for application purposes. Although there are several excellent examples of hydrophilic–hydrophobic bifunctional stabilizers that can work in both aqueous and organic media,3 in most cases MNPs protected by a certain stabilizer are only soluble in a limited number of solvents. Thus the lack of the tunable solubility of the MNPs has become an obstacle for their applications.3ePoly-1-vinyl-2-pyrrolidone (PVP), for example, is one of the most powerful stabilizers for MNP preparation. The applications of the PVP stabilized MNPs, however, are largely limited to water4 and alcohol5 media due to the insoluble nature of PVP in non-protic solvents. Previously, we developed a novel polymer by copolymerization of PVP monomer and an ionic monomer which became applicable in ionic liquids (ILs).6 Nevertheless, a versatile strategy for the application of PVP type stabilizers is still lacking. We report herein the development of a polarity adjustable PVP family by molecular engineering of PVP to expand the range of solvents used for the preparation and application of MNPs.
N-Vinyl-2-pyrrolidone (NVP), the monomer of PVP, can be functionalized first by deprotonation at position 3 by a strong base to form a carbanion followed by a nucleophilic reaction.7 Reaction of NVP with lithium diisopropylamide (LDA) and then with alkyl bromide gave two products, 3-alkyl-1-vinyl-2-pyrrolidone and 3,3-dialkyl-1-vinyl-2-pyrrolidone (see Scheme 1). Free radical polymerization of 3-alkyl-1-vinyl-2-pyrrolidone yielded the corresponding polymer whereas 3,3-dialkyl-1-vinyl-2-pyrrolidone was resistant to polymerization under identical conditions. Thus, a series of poly-3-ethyl-1-vinyl-2-pyrrolidone (C2-PVP), poly-3-butyl-1-vinyl-2-pyrrolidone (C4-PVP), poly-3-hexyl-1-vinyl-2-pyrrolidone (C6-PVP) and poly-3-octyl-1-vinyl-2-pyrrolidone (C8-PVP) was obtained. Their molecular weights were found to be approximately 14–19 kDa and their structures verified by elemental analysis, NMR and IR techniques (see ESI† for data and spectra). Their decomposition temperatures under both inert and oxidative atmosphere are higher than 350 °C, as demonstrated by TGA analysis (see ESI,† Fig. S3). These four polymers, together with commercially available PVP, formed a family of MNPs stabilizers (hereinafter referred as Cn-PVP) with similar capability of protecting MNPs but distinctive solubility behaviors.
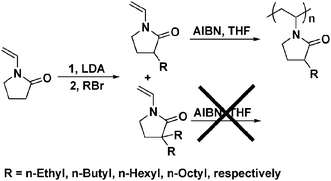 | ||
| Scheme 1 Synthetic strategy for Cn-PVP.‡ | ||
To investigate the possibility of using alkyl modified PVP as MNP stabilizers and the behaviors of the prepared MNPs, soluble Rh MNPs protected by Cn-PVP were prepared using RhCl3 as the metal precursor and H2 as the reducing agent (see ESI† for synthetic details). The micrographs of the Rh MNPs are shown in Fig. 1. Relatively narrow unimodal size distributions with a diameter centered at approximately 3 nm are observed for PVP, C2-PVP, C4-PVP and C6-PVP protected Rh MNPs. For C8-PVP protected Rh MNPs the size distribution is slightly wider and the average diameter is approximately 1 nm larger. The Rh nanoparticles were further characterized by FT-IR spectroscopy using CO as the probe. For all the five samples, three peaks in the region of 1980–2080 cm−1 were observed (see ESI,† Fig. S6), which can be attributed to Rh(CO)2 (∼2072 and ∼2000 cm−1) and Rh–CO (2017 cm−1), respectively.8 The similarity of their adsorption curves indicates the surface coordination properties of the five nanoparticles are similar.
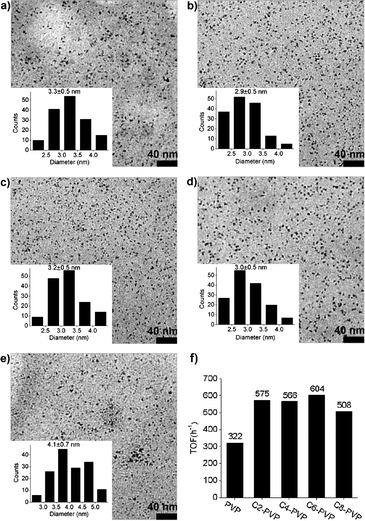 | ||
| Fig. 1 TEM micrographs and size distributions of Rh MNPs stabilized by PVP and Cn-PVP: (a) Rh-PVP; (b) Rh-C2-PVP; (c) Rh-C4-PVP; (d) Rh-C6-PVP; (e) Rh-C8-PVP. (f) TOF of Rh stabilized by the PVP family towards toluene hydrogenation (80 °C, 3 MPa H2, ethanol 10 mL, toluene 1 mL, Rh 0.05 mmol). | ||
The current work focuses on the systematic solubility modulation of the stabilizer so that reaction media can be chosen rationally. As such, the solubility of the MNPs-Cn-PVP as a function of aliphatic chain length was of particular interest. The solubility of various Rh MNPs in thirteen common solvents, ranging in polarity from water to hexane, was examined (see Fig. 2). Rh-PVP was only soluble in water, ethanol and partially soluble in acetic acid and acetonitrile, being completely insoluble in all the other solvents. As the length of the side chain increases, the Rh-Cn-PVP gradually became more nonpolar and insoluble in water. However, their solubility in less polar solvents is greatly improved; for example, Rh-C2-PVP dissolved in THF and halogenated alkanes. Rh-C6-PVP exhibited the best solubility behavior and was soluble in 10 solvents, including hexane. It is interesting to note that the solubility behavior of the Cn-PVP family protected Rh MNPs in acetonitrile and acetone did not obey monotonic trends. Rh-PVP can not be dissolved in either of the two solvents. As the side alkyl chain length increases, Rh-Cn-PVP became first soluble and then insoluble in both cases. The partition coefficients of the various Rh-Cn-PVP MNPs were also tested using hexane and water (see ESI,† Fig. S4). Rh-PVP was completely dissolved in the bottom aqueous layer while Rh-C8-PVP was found exclusively in the hexane phase, displaying a dramatic solubility change in the series of Rh-Cn-PVP MNPs.
 | ||
| Fig. 2 Solubility of Rh MNPs stabilized by the PVP family in thirteen solvents (from top to bottom: Rh-PVP, Rh-C2-PVP, Rh-C4-PVP, Rh-C6-PVP and Rh-C8-PVP; Rh 5 mM, Rh : Cn-PVP = 1 : 10, pictures taken after one month storing). | ||
Arene hydrogenation was considered to be an ideal model reaction to evaluate the catalytic activity of various Rh MNPs. Since ethanol is the only solvent that can dissolve (or partially dissolve) all five Rh-Cn-PVPs, toluene hydrogenation was carried out in ethanol at 80 °C and 3 MPa H2 by Rh-Cn-PVP MNPs (Fig. 1(f)). The turnover frequency (TOF) of Rh-PVP towards toluene hydrogenation was 322 h−1, similar to that of Rh MNPs stabilized by PVP-IL copolymers.6aRh-Cn-PVP (n = 2, 4, 6) exhibited much higher activity, with TOF values approximately twice that of Rh-PVP MNPs. Considering that the TEM and IR analysis of the Rh MNPs were similar, the different activity may arise from the decreased polarity of the Rh-Cn-PVP which makes toluene more accessible to the surface of the MNPs. The TOF value of Rh-C8-PVP MNPs decreased slightly with respect to Rh-Cn-PVP (n = 2, 4, 6), which could be due, at least in part, to the increased diameter of the Rh-C8-NPs or to the lower solubility of Rh-C8-PVP in ethanol compared to the others (vide supra).
TEM and CO adsorption analysis, together with the solubility tests and the catalytic activity of the Rh-Cn-PVP, suggest that (a) the solubility of the MNPs prepared by employing Cn-PVP as stabilizer is adjustable, enabling optional choice of a solvent as the reaction media; (b) other properties of the MNPs (at least for Rh), such as the size, the chemical environment and the catalytic activity are similar.
In a demonstration of the versatility of Cn-PVP based MNPs, other metals and reducing agents, as well as solvents with different polarity, were used (see ESI,† Fig. S7 for TEM pictures and EDX analysis). Not surprisingly, Ru, Pt and Pd (entries 1–3, Table 1) MNPs were successfully obtained by classical methods employing EtOH or NaBH4 as the reductant. Further utilizing the tunable solubility of the novel Cn-PVP stabilizers, LiAlH4 was employed for the preparation of Ag MNPs in dioxane (entry 4, Table 1). Co MNPs were readily prepared by thermal decomposition of Co2(CO)8 in the presence of C8-PVP in toluene or squalene (entries 5 and 6, Table 1). From this it is clear that functionalizing PVP with an alkyl chain not only endows MNPs with adjustable solubility but also expands the range of possible reagents (both reductants and metal precursors) and as a result the scope of PVP based MNPs formation and catalysis.
| Entry | Metal | Precursor | Stabilizer | Solvent | Prep. method | Size/nm |
|---|---|---|---|---|---|---|
| a MNPs were found to stick to each other and as a result the size distribution was not determined. | ||||||
| 1 | Ru | RuCl3 | C2-PVP | EtOH–H2O | NaBH4 as reductant | ∼5–7a |
| 2 | Pd | PdCl2 | C4-PVP | EtOH | EtOH as reductant | 5.0 ± 1.2 |
| 3 | Pt | H2PtCl6 | C4-PVP | EtOH | NaBH4 as reductant | 2.3 ± 0.3 |
| 4 | Ag | AgOAc | C6-PVP | Dioxane | LiAlH4 as reductant | 4.0 ± 1.1 |
| 5 | Co | Co2(CO)8 | C8-PVP | Toluene | Thermal decomp. | 15–30a |
| 6 | Co | Co2(CO)8 | C8-PVP | Squalane | Thermal decomp. | 15–30a |
Recently, several Fischer–Tropsch (F–T) processes based on soluble Ru and Co MNPs have been established.9 However, these researches were conducted in water and ILs, which are not the conventional solvents applied in the slurry reactor for F–T reaction. A quasi-homogeneous process that uses high boiling point alkanes as solvent, as currently practised in industry, has not yet been developed. With this in mind, we tested the F–T synthesis activity over Co MNPs derived from the thermal decomposition of Co2(CO)8 in squalane and stabilized by C8-PVP. Co-C8-PVP exhibited F–T reaction activity at temperatures higher than 170 °C with the TOF value increasing with temperature. The reaction of syngas (20 atm, H2–CO 2 : 1) over the Co-C8-PVP at 200 °C for 12 h gave an average TOF value of 1.3, of the same order of magnitude as the value observed for supported cobalt catalysts and higher than that of Co MNPs in ILs (see ESI,† Table S1). However, the MNPs were not stable and after reaction, Co MNPs were partially precipitated and Co black was found in the autoclave. Nevertheless, this is the first example of a F–T process catalyzed by soluble Co MNPs in paraffin solvents, demonstrating that MNPs stabilized by the hydrophobic C8-PVP can be applied in nonpolar solvents for catalysis.
In summary, we have developed a general strategy for the preparation of solubility adjustable MNPs by selecting appropriate Cn-PVP as the stabilizer. We believe the current work should broaden the applications of MNPs protected by PVP-like polymers. For example, they can be, in principle, applied as catalysts for novel biphasic reactions. Moreover, the modified PVP family for MNPs preparation demonstrates that there is great potential to improve quasi-homogeneous catalysis by tailoring the stabilizers. Further examples involving steric, electronic modifications and bi-functional stabilizers are currently under development in this laboratory.
This work was supported by the National Science Foundation of China (Projects 20773005 and 20533010). The authors thank Mr Ryan Dykeman for useful discussions.
Notes and references
- (a) G. Schmid, Nanoparticles: From Theory to Application, Wiley, New York, 2004 Search PubMed; (b) M. C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293 CrossRef CAS; (c) T. H. Lee, J. I. Gonzalez, J. Zheng and R. M. Dickson, Acc. Chem. Res., 2005, 38, 534 CrossRef CAS; (d) N. L. Rosi and C. A. Mirkin, Chem. Rev., 2005, 105, 1547 CrossRef CAS; (e) D. Astruc, Nanoparticles and Catalysis, VCH, Weinheim, 2008 Search PubMed; (f) A. J. Baca, J. H. Ahn, Y. Sun, M. A. Meitl, E. Menard, H. S. Kim, W. M. Choi, D. H. Kim, Y. Huang and J. A. Rogers, Angew. Chem., Int. Ed., 2008, 47, 5524 CrossRef CAS.
- For examples see: (a) A. Roucoux, J. Schulz and H. Patin, Chem. Rev., 2002, 102, 3757 CrossRef CAS; (b) J. A. Widegren and R. G. Finke, J. Mol. Catal. A: Chem., 2003, 191, 187 CrossRef CAS; (c) J. Grunes, A. Zhu and G. A. Somorjai, Chem. Commun., 2003, 2257 RSC; (d) B. L. Cushing, V. L. Kolesnichenko and C. J. O’Connor, Chem. Rev., 2004, 104, 3893 CrossRef CAS; (e) C. Burda, X. B. Chen, R. Narayanan and M. A. El-Sayed, Chem. Rev., 2005, 105, 1025 CrossRef CAS; (f) D. Astruc, F. Lu and J. R. Aranzaes, Angew. Chem., Int. Ed., 2005, 44, 7852 CrossRef CAS; (g) T. J. Geldbach, D. B. Zhao, N. C. Castillo, G. Laurenczy, B. Weyershausen and P. J. Dyson, J. Am. Chem. Soc., 2006, 128, 9773 CrossRef CAS; (h) D. B. Zhao, Z. F. Fei, W. H. Ang and P. J. Dyson, Small, 2006, 2, 879 CrossRef CAS; (i) P. Migowski and J. Dupont, Chem.–Eur. J., 2007, 13, 32 CrossRef; (j) R. Narayanan and M. A. El-Sayed, Top. Catal., 2008, 47, 15 CrossRef CAS; (k) G. A. Somorjai and J. Y. Park, Top. Catal., 2008, 49, 126 CrossRef CAS.
- (a) S. Minko, S. Patil, V. Datsyuk, F. Simon, K. J. Eichhorn, M. Motornov, D. Usov, I. Tokarev and M. Stamm, Langmuir, 2002, 18, 289 CrossRef CAS; (b) B. L. V. Prasad, S. K. Arumugarn, T. Bala and M. Sastry, Langmuir, 2005, 21, 822 CrossRef CAS; (c) S. C. Zimmerman, J. R. Quinn, E. Burakowska and R. Haag, Angew. Chem., Int. Ed., 2007, 46, 8164 CrossRef CAS; (d) J. Keilitz, M. R. Radowski, J. D. Marty, R. Haag, F. Gauffre and C. Mingotaud, Chem. Mater., 2008, 20, 2423 CrossRef CAS; (e) A. M. Smith and S. Nie, Angew. Chem., Int. Ed., 2008, 47, 9916 CrossRef CAS.
- For examples, see: (a) N. Yan, C. Zhao, C. Luo, P. J. Dyson, H. C. Liu and Y. Kou, J. Am. Chem. Soc., 2006, 128, 8714 CrossRef CAS; (b) C. X. Mao, H. Z. Wang, X. D. Mu and Y. Kou, J. Catal., 2007, 250, 25 CrossRef CAS.
- For examples, see: (a) X. Yang, N. Yan, Z. F. Fei, R. M. Crespo-Quesada, G. Laurenczy, L. Kiwi-Minsker, Y. Kou, Y. D. Li and P. J. Dyson, Inorg. Chem., 2008, 47, 7444 CrossRef CAS; (b) L. He, H. Liu, C. X. Xiao and Y. Kou, Green Chem., 2008, 10, 619 RSC.
- (a) X. D. Mu, J. Q. Meng, Z. C. Li and Y. Kou, J. Am. Chem. Soc., 2005, 127, 9694 CrossRef CAS; (b) C. Zhao, H. Z. Wang, N. Yan, C. X. Xiao, X. D. Mu, P. J. Dyson and Y. Kou, J. Catal., 2007, 250, 33 CrossRef CAS.
- (a) L. A. White, S. Jonson, C. E. Hoyle and L. J. Mathias, Polymer, 1999, 40, 6597 CrossRef CAS; (b) L. A. White, C. E. Hoyle, S. Jonsson and L. J. Mathias, J. Polym. Sci., Part A: Polym. Chem., 2002, 40, 694 CrossRef CAS.
- M. R. Mucalo and R. P. Cooney, Chem. Mater., 1991, 3, 1081 CrossRef CAS.
- (a) C. X. Xiao, Z. P. Cai, T. Wang, Y. Kou and N. Yan, Angew. Chem., Int. Ed., 2008, 47, 746 CrossRef CAS; (b) D. O. Silva, J. D. Scholten, M. A. Gelesky, S. R. Teixeira, A. C. B. Dos Santos, E. F. Souza-Aguiar and J. Dupont, ChemSusChem, 2008, 1, 291 CrossRef CAS; (c) M. Scariot, D. O. Silva, J. D. Scholten, G. Machado, S. R. Teixeira, M. A. Novak, G. Ebeling and J. Dupont, Angew. Chem., Int. Ed., 2008, 47, 9075 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed synthetic procedures and characterization of the Cn-PVP and Cn-PVP protected MNPs, as well as instrumentation and catalysis. See DOI: 10.1039/b905625d |
| ‡ All organic synthesis was handled with standard Schlenk techniques. |
| This journal is © The Royal Society of Chemistry 2009 |
