Digital fluorescent pH sensors†
Seiichi
Uchiyama
* and
Yumi
Makino
Graduate School of Pharmaceutical Sciences, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan. E-mail: seiichi@mol.f.u-tokyo.ac.jp; Fax: +81 3 5841 4768; Tel: +81 3 5841 4768
First published on 10th March 2009
Abstract
We designed polymeric sensors that created a digital-type fluorescence response to pH variation in an aqueous solution.
An important challenge in developing new chemical ion sensors is to design them with the ability to generate a digital-type output signal1 that can be used in binary switching systems as highly sensitive ion indicators2 and in molecular devices3 such as data storage tools.4 Until now, an inner filter effect2 and a dynamic multichromophore array5 have been utilized to sharply respond to ion concentration variation; however, the inflexible requirements of these methods limit their widespread use. In this communication, we demonstrate digital-type fluorescent pH sensors based on the incorporation of a water-sensitive fluorophore into a pH-responsive polymer.6,7 The fluorescence output of these polymeric pH sensors is switched in a significantly narrow pH range (i.e., within almost a unit). In addition, their functions, such as operating pH range and switching direction (i.e., on–off and off–on actions), can be easily tuned by structural modification of a proton receptor. It is noteworthy that many digital signal transductions occur in living organisms, in which the digital processors are biological versions of macromolecules.8
Fig. 1a depicts the chemical structure of a digital fluorescent pH sensor, 1 (Mn = 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600, Mw/Mn = 2.31), which was prepared by random copolymerization of N-isopropylacrylamide (NIPAM), N,N-dimethylaminopropylacrylamide (DMAPAM) bearing an amino group as a proton receptor, and fluorescent N-{2-[(7-N,N-dimethylaminosulfonyl)-2,1,3-benzoxadiazol-4-yl](methyl)amino}ethyl-N-methylacrylamide (DBD-AA) (see ESI† for experimental details). The use of a similar copolymer as a pH sensor has already been reported by Onoda et al.9 Nevertheless, the present communication is the first to discuss the digital sensory behavior of these copolymers . Fig. 1b and c indicate the relationship between the fluorescence intensity of 1 in an aqueous solution and medium pH. As depicted in Fig. 1b (filled circles), 1 in aqueous solution emitted 6.6-fold stronger fluorescence under basic conditions (pH > 9) than under acidic conditions (pH < 8) at 50 °C. Under the latter conditions, 1 assumes a hydrated open form due to the high hydrophilicity of the protonated DMAPAM units, and solventwater molecules approach the DBD-AA units7e to quench their fluorescence. On the other hand, 1 exists in a dehydrated globular form in a basic solution because of the hydrophobic interactions between the NIPAM and unprotonated DMAPAM units, where the DBD-AA units fluoresce at a location far from the water molecules. It should be noted that the fluorescence response of 1 disappeared when the solution temperature was decreased to 20 °C (see open circles in Fig. 1b). This temperature-specific function is a typical feature of polymeric pH sensors.9,10 At the lower temperatures, hydration by the solvent is a dominant interaction, even under basic conditions, such that 1 never assumes the dehydrated globular form.11
600, Mw/Mn = 2.31), which was prepared by random copolymerization of N-isopropylacrylamide (NIPAM), N,N-dimethylaminopropylacrylamide (DMAPAM) bearing an amino group as a proton receptor, and fluorescent N-{2-[(7-N,N-dimethylaminosulfonyl)-2,1,3-benzoxadiazol-4-yl](methyl)amino}ethyl-N-methylacrylamide (DBD-AA) (see ESI† for experimental details). The use of a similar copolymer as a pH sensor has already been reported by Onoda et al.9 Nevertheless, the present communication is the first to discuss the digital sensory behavior of these copolymers . Fig. 1b and c indicate the relationship between the fluorescence intensity of 1 in an aqueous solution and medium pH. As depicted in Fig. 1b (filled circles), 1 in aqueous solution emitted 6.6-fold stronger fluorescence under basic conditions (pH > 9) than under acidic conditions (pH < 8) at 50 °C. Under the latter conditions, 1 assumes a hydrated open form due to the high hydrophilicity of the protonated DMAPAM units, and solventwater molecules approach the DBD-AA units7e to quench their fluorescence. On the other hand, 1 exists in a dehydrated globular form in a basic solution because of the hydrophobic interactions between the NIPAM and unprotonated DMAPAM units, where the DBD-AA units fluoresce at a location far from the water molecules. It should be noted that the fluorescence response of 1 disappeared when the solution temperature was decreased to 20 °C (see open circles in Fig. 1b). This temperature-specific function is a typical feature of polymeric pH sensors.9,10 At the lower temperatures, hydration by the solvent is a dominant interaction, even under basic conditions, such that 1 never assumes the dehydrated globular form.11
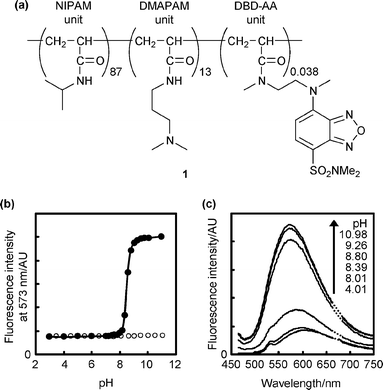 | ||
| Fig. 1 (a) Chemical structure of 1. (b) Fluorescence responses of 1 (0.01 w/v%) to pH variation at 50 °C (●) and 20 °C (○) in a Britton–Robinson buffer. Excitation: 450 nm. The fluorescence quantum yield was 0.035 at 50 °C and pH 3. (c) Fluorescence spectra of 1 at 50 °C. Conditions are the same as in (b). | ||
In comparison to the conventional 8-hydroxypyrene-1,3,6-trisulfonate (HPTS) pH sensor12 (Fig. 2), the fluorescence switching of 1 was completed in a narrower pH range (within about one unit from pH 8 to 9). In order to quantify the similarity of the fluorescent pH sensor output to a perfect digital signal, the fluorescence signals were fitted to the following proposed equation by assuming that the fluorescence intensity of 1 was linear to the proportion of the protonated DMAPAM units as the fluorescence intensity of HPTS is linear to the proportion of the phenolate form:
| −log[(FImax − FI)/(FI − FImin)] = a(pH − pKa) | (1) |
 | ||
| Fig. 2 (a) Chemical structure of HPTS. (b) Fluorescence responses of HPTS (1 μM) to pH variation in a Britton–Robinson buffer at 25 °C. Excitation: 454 nm. | ||
The mechanism of this sharp response is related to a decrease in the Lewis basicity of the receptor amine in the DMAPAM units with a conformational change occurring in the molecule of 1 from the open form at a low pH to the globular form at a high pH. In general, the pKa value of the protonated amine receptors decreases by more than one unit in a hydrophobic environment due to the destabilization of the protonated receptors by dielectric effects.15 In the case of 1, a pKa value for the protonated DMAPAM units at the lower pH (<8) should be nearly identical to that in water (pKa = 9.316), whereas the pKa value at the higher pH (>9) can be estimated to be less than 8.3. In the intermediate pH range between 8 and 9, the pKa value for the protonated DMAPAM units gradually decreases from 9.3 to less than 8.3 as a function of increasing pH because the structural change of 1 to the globular form makes the local environments near the DMAPAM units hydrophobic (cf., apparent pKa for 1 obtained from eqn (1) was an intermediate value of 8.68, as discussed in the former paragraph). Thus, the response curve of 1 in the fluorescence intensity–pH diagram becomes steeper than that of conventional pH sensors working with a pKa value that remains unchanged as the pH changes.17
In order to confirm that the fluorescence response of 1 originated from the combination of the NIPAM, DMAPAM, and DBD-AA units, the fluorescence properties of control compounds (Fig. 3) were studied in a broad temperature range between 20 and 70 °C. Neither DBD-IA7e (a model fluorophore of the DBD-AA unit) nor the copolymer 2, consisting of only DMAPAM and DBD-AA units (i.e., without NIPAM units) (Mn = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700, Mw/Mn = 1.92), was sensitive to pH variation (data not shown). Indeed, the insensitivity of 2 clearly demonstrates that the NIPAM units were required for 1 to give the digital-type output signal. Most likely, the NIPAM units of 1 maintained a hydrophobic–hydrophilic balance that allowed for a conformational change between the open and globular forms with pH variation. In other words, the excess DMAPAM units in 2 made the copolymer too hydrophilic to assume a globular form, even under basic conditions. In this sense, replacement of the NIPAM units by more hydrophobic units could decrease the functional temperature of the copolymer from 50 °C.9
700, Mw/Mn = 1.92), was sensitive to pH variation (data not shown). Indeed, the insensitivity of 2 clearly demonstrates that the NIPAM units were required for 1 to give the digital-type output signal. Most likely, the NIPAM units of 1 maintained a hydrophobic–hydrophilic balance that allowed for a conformational change between the open and globular forms with pH variation. In other words, the excess DMAPAM units in 2 made the copolymer too hydrophilic to assume a globular form, even under basic conditions. In this sense, replacement of the NIPAM units by more hydrophobic units could decrease the functional temperature of the copolymer from 50 °C.9
![Chemical structures of DBD-IA (N,2-dimethyl-N-(2-{methyl[7-(dimethylsulfamoyl)-2,1,3-benzoxadiazol-4-yl]amino}ethyl)propanamide) and 2.](/image/article/2009/CC/b900889f/b900889f-f3.gif) | ||
| Fig. 3 Chemical structures of DBD-IA (N,2-dimethyl-N-(2-{methyl[7-(dimethylsulfamoyl)-2,1,3-benzoxadiazol-4-yl]amino}ethyl)propanamide) and 2. | ||
The functional pH range of our digital fluorescent pH sensor can easily be tuned by modifying the chemical structure of the ionizable units. For instance, when N-(3-morpholin-4-ylpropyl)acrylamide (MPAM) units with a weaker proton receptor (pKa for the conjugate acid of MPAM is 7.0 in water16) were incorporated into the copolymer instead of the DMAPAM units, the functional pH range of the resultant copolymer 3 (Fig. 4, Mn = 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Mw/Mn = 3.04) was shifted to the acidic region (apparent pKa = 6.16 ± 0.03), and the output signal remained digital (a for eqn (1) = 2.54 ± 0.05), as indicated in Fig. 5a. In contrast, the use of a stronger receptor unit, N-[3-(diethylamino)propyl]acrylamide (DEAPAM) (pKa for the conjugate acid of DEAPAM is 10.3 in water16), as in copolymer 4 (Fig. 4, Mn = 33
000, Mw/Mn = 3.04) was shifted to the acidic region (apparent pKa = 6.16 ± 0.03), and the output signal remained digital (a for eqn (1) = 2.54 ± 0.05), as indicated in Fig. 5a. In contrast, the use of a stronger receptor unit, N-[3-(diethylamino)propyl]acrylamide (DEAPAM) (pKa for the conjugate acid of DEAPAM is 10.3 in water16), as in copolymer 4 (Fig. 4, Mn = 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800, Mw/Mn = 2.25), moved the functional pH to the basic region (apparent pKa = 9.12 ± 0.05 and a = 4.75 ± 0.44), as displayed in Fig. 5b. In addition, an acrylic acid (AA) unit (pKa of isobutyric acid is 4.9 in water18) can also be used as an ionizable unit. Then, the fluorescence response of copolymer 5 (Fig. 4, Mn = 38
800, Mw/Mn = 2.25), moved the functional pH to the basic region (apparent pKa = 9.12 ± 0.05 and a = 4.75 ± 0.44), as displayed in Fig. 5b. In addition, an acrylic acid (AA) unit (pKa of isobutyric acid is 4.9 in water18) can also be used as an ionizable unit. Then, the fluorescence response of copolymer 5 (Fig. 4, Mn = 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, Mw/Mn = 3.17), composed of NIPAM, AA, and DBD-AA units, was reversed from that of 1; that is, it emitted a stronger fluorescence under acidic conditions (Fig. 5c, apparent pKa = 5.17 ± 0.13 and |a| = 4.57 ± 0.59). This is because 5 assumes a globular form at a low pH wherein a hydrophobic interaction is induced between NIPAM and the unionized AA units.
100, Mw/Mn = 3.17), composed of NIPAM, AA, and DBD-AA units, was reversed from that of 1; that is, it emitted a stronger fluorescence under acidic conditions (Fig. 5c, apparent pKa = 5.17 ± 0.13 and |a| = 4.57 ± 0.59). This is because 5 assumes a globular form at a low pH wherein a hydrophobic interaction is induced between NIPAM and the unionized AA units.
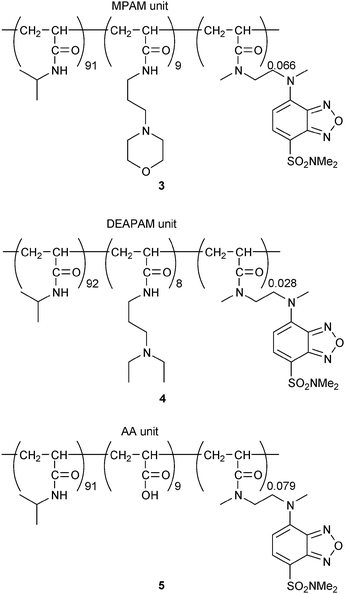 | ||
| Fig. 4 Chemical structures of 3–5. | ||
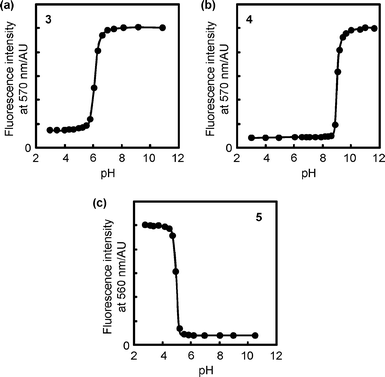 | ||
| Fig. 5 Fluorescence responses of (a) 3 at 50 °C, (b) 4 at 50 °C, and (c) 5 at 45 °C to pH variation in a Britton–Robinson buffer. Concentration: 0.01 w/v%. Excitation: 450 nm. | ||
In summary, we have developed digital fluorescent pH sensors based on the copolymers of NIPAM, an ionizable acrylic acid derivative (DMAPAM, MPAM, DEAPAM, or AA), and DBD-AA. These pH sensors switch their output signals in a narrow pH range (within almost one unit). In addition to changing the functional pH ranges, the switching direction can be changed by adopting different ionizable units. The polymeric design of our new digital sensors also offers advantages in modifying the fluorescence color7a,7f and attaching its function to bulk materials.19 More examples and a further study on the functional mechanism are needed to develop these digital fluorescent pH sensors further.
We thank Ms. C. Gota and Ms. M. Onoda for valuable comments and discussions. S.U. thanks the Foundation for Promotion of Material Science and Technology of Japan (MST Foundation) for financial support.
Notes and references
- K. Szaciłowski, Chem. Rev., 2008, 108, 3481–3548 CrossRef CAS.
- G. Gabor and D. R. Walt, Anal. Chem., 1991, 63, 793–796 CrossRef CAS.
- V. Balzani, A. Credi and M. Venturi, Molecular Devices and Machines, Wiley-VCH, Weinheim, 2nd edn, 2008 Search PubMed.
- M. Irie, T. Fukaminato, T. Sasaki, N. Tamai and T. Kawai, Nature, 2002, 420, 759–760 CrossRef CAS.
- J. A. Riddle, X. Jiang, J. Huffman and D. Lee, Angew. Chem., Int. Ed., 2007, 46, 7019–7022 CrossRef CAS.
- Some pH-responsive gels encounter a digital-type phase transition with changing environmental pH. See: (a) T. Tanaka, D. Fillmore, S.-T. Sun, I. Nishio, G. Swislow and A. Shah, Phys. Rev. Lett., 1980, 45, 1636–1639 CrossRef CAS; (b) R. A. Siegel and B. A. Firestone, Macromolecules, 1988, 21, 3254–3259 CrossRef CAS.
- The relevant combination of a water-sensitive fluorophore and a thermo-responsive polymer has been applied to fluorescent thermometers showing digital-type output signals. See: (a) S. Uchiyama, Y. Matsumura, A. P. de Silva and K. Iwai, Anal. Chem., 2003, 75, 5926–5935 CrossRef CAS; (b) S. Uchiyama, Y. Matsumura, A. P. de Silva and K. Iwai, Anal. Chem., 2004, 76, 1793–1798 CrossRef CAS; (c) C. Gota, S. Uchiyama and T. Ohwada, Analyst, 2007, 132, 121–126 RSC; (d) Y. Shiraishi, R. Miyamoto, X. Zhang and T. Hirai, Org. Lett., 2007, 9, 3921–3924 CrossRef CAS; (e) C. Gota, S. Uchiyama, T. Yoshihara, S. Tobita and T. Ohwada, J. Phys. Chem. B, 2008, 112, 2829–2836 CrossRef CAS; (f) Y. Shiraishi, R. Miyamoto and T. Hirai, Langmuir, 2008, 24, 4273–4279 CrossRef CAS.
- T. Tian, A. Harding, K. Inder, S. Plowman, R. G. Parton and J. F. Hancock, Nat. Cell Biol., 2007, 9, 905–914 CrossRef CAS.
- M. Onoda, S. Uchiyama and T. Ohwada, Macromolecules, 2007, 40, 9651–9657 CrossRef CAS.
- (a) S. Beltran, J. P. Baker, H. H. Hooper, H. W. Blanch and J. M. Prausnitz, Macromolecules, 1991, 24, 549–551 CrossRef CAS; (b) G. Chen and A. S. Hoffman, Nature, 1995, 373, 49–52 CrossRef CAS; (c) S. Uchiyama, N. Kawai, A. P. de Silva and K. Iwai, J. Am. Chem. Soc., 2004, 126, 3032–3033 CrossRef CAS.
- X. Yin, A. S. Hoffman and P. S. Stayton, Biomacromolecules, 2006, 7, 1381–1385 CrossRef CAS.
- O. S. Wolfbeis, E. Fürlinger, H. Kroneis and H. Marsoner, Fresenius′ Z. Anal. Chem., 1983, 314, 119–124 CrossRef CAS.
- (a) L. J. Henderson, Am. J. Physiol., 1908, 21, 173–179 Search PubMed; (b) K. A. Hasselbalch, Biochem. Z., 1917, 78, 112–144 Search PubMed; (c) H. N. Po and N. M. Senozan, J. Chem. Educ., 2001, 78, 1499–1503 CrossRef CAS.
- (a) F. M. Raymo, Adv. Mater., 2002, 14, 401–414 CrossRef CAS; (b) U. Pischel, Angew. Chem., Int. Ed., 2007, 46, 4026–4040 CrossRef CAS; (c) A. P. de Silva and S. Uchiyama, Nat. Nanotechnol., 2007, 2, 399–410 Search PubMed.
- (a) M. S. Fernández and P. Fromherz, J. Phys. Chem., 1977, 81, 1755–1761 CrossRef; (b) C. J. Drummond, F. Grieser and T. W. Healy, J. Chem. Soc., Faraday Trans. 1, 1989, 85, 521–535 RSC; (c) S. Uchiyama, K. Iwai and A. P. de Silva, Angew. Chem., Int. Ed., 2008, 47, 4667–4669 CrossRef CAS.
- P. G. Righetti, E. Gianazza, C. Gelfi, M. Chiari and P. K. Sinha, Anal. Chem., 1989, 61, 1602–1612 CrossRef CAS.
- A related pKa shift has been discussed on a drastic ionization of nitrated poly(4-hydroxystyrene) with increasing pH. See: D. Westover, W. R. Seitz and B. K. Lavine, Microchem. J., 2003, 74, 121–129 Search PubMed.
- K. Kashiwagi, R. Sugise, T. Shimakawa, T. Matuura and M. Shirai, J. Mol. Catal. A: Chem., 2007, 264, 9–16 CrossRef CAS.
- A. P. Herrera, M. Rodríguez, M. Torres-Lugo and C. Rinaldi, J. Mater. Chem., 2008, 18, 855–858 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details. See DOI: 10.1039/b900889f |
| This journal is © The Royal Society of Chemistry 2009 |
