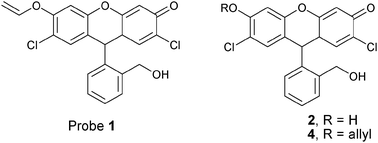
A chemodosimeter approach to fluorescent sensing and imaging of inorganic and methylmercury species†
Mithun
Santra
a,
Dowook
Ryu
a,
Amrita
Chatterjee
a,
Sung-Kyun
Ko
b,
Injae
Shin
*b and
Kyo Han
Ahn
*a
aDepartment of Chemistry and Center for Electro-Photo Behaviors in Advance Molecular Systems, POSTECH, San 31 Hyoja-dong, Pohang, 790-784, Republic of Korea. E-mail: ahn@postech.ac.kr
bDepartment of Chemistry, Yonsei University, Seoul 120-749, Republic of Korea. E-mail: injae@yonsei.ac.kr
First published on 16th March 2009
Abstract
A highly sensitive fluorescent turn-on probe specific for methylmercury species as well as inorganic mercury ions has been developed on the basis of mercury ion-promoted hydrolysis of a fluorescein -derived arylvinylether.
Mercury is a highly poisonous element and widespread pollutant, occurring from natural and anthropogenic sources. Organic forms of mercury, typically, methylmercury species (CH3HgX; X = halides, etc.) are much more toxic than inorganic mercury species (HgX2).1 Because of their lipid solubility, methylmercury species readily cross the blood–brain barrier, accumulate in the brain, and cause damage to the central nervous system,1,2 as well as other organs. The epidemics of Minamata Bay in Japan demonstrated the fatal threat of methylmercury to human health.3
A variety of fluorescent molecular probes for the detection of mercury ions with ease and high sensitivity have flourished in recent years.4 Surprisingly, most of these probes only sense inorganic mercury ions and those that sense methylmercury are rare.5 Furthermore, none of the fluorescent probes can be applied to evaluate the accumulation of methylmercury in living species. For example, the rhodamine-based fluorescent chemodosimeter reported by Tae and co-workers shows high sensitivity and selectivity towards inorganic mercury ions, and has been used to monitor them both in cells and in a living vertebrate organism. However, the chemodosimeter showed a weak response to CH3HgCl in contrast to HgCl2.4g,k
One plausible reason for the low sensitivity of the heteroatom-based molecular probes towards methylmercury may arise from the weak molecular interactions between them. To develop a fluorescent probe for the notoriously poisonous methylmercury ions, we have designed a chemodosimeter based on a chemical reaction that avoids the popular mercury ion-coordination to heteroatoms.6 The chemodosimeter thus designed is fluorescein-based vinyl ether, 1, which reacts only with organomercury and inorganic mercury ions in the presence of other metal ions, and shows a “turn-on”-type fluorescent response with high sensitivity. The fluorescent imaging of mammalian cells and vertebrate organisms treated with methylmercury is also demonstrated for the first time.
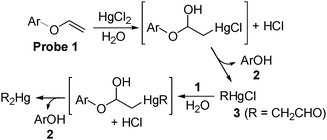
The design of probe 1 is based on the mercury ion-promoted hydrolysis reaction of vinylethers: the oxymercuration of vinyl ether 1 generates the corresponding hemiacetal intermediate, which undergoes fragmentation into alcohol 2 and aldehyde 3 (Scheme 1).
Such a mercury ion-promoted hydrolysis of vinyl ether 1 is also expected to occur with methylmercury species (CH3HgX), because a similar oxymercuration intermediate is envisioned on the basis of the reaction mechanism. Because vinyl ether 1 is very weakly fluorescent, but alcohol 2 is highly fluorescent, we may realize fluorescent turn-on sensing of methylmercury as well as inorganic mercury ions in aqueous media.
The required vinyl ether 1 was directly synthesized from known compound 4 through deallylation and vinylation steps.7
A solution of probe 1 (5.0 μM) in PBSbuffer (pH 7.4) containing 5% DMSO became strongly fluorescent upon addition of HgCl2 (Fig. 1(a)), as it is converted to fluorescein 2 (ΦF = 0.89)7a through hydrolysis. The generation of 2 was confirmed by isolation and characterization of the final product after titration . Importantly, the titration of probe 1 with HgCl2 (from 0 to 1.0 equiv.) showed saturation behavior at 0.5 equiv. of HgCl2 (Fig. 1(b)), which can be explained by the mechanism in Scheme 1. The mechanism suggests that an organomercury species such as 3 also promotes the hydrolysis. Certainly, probe 1 shows fluorescence recovery upon treatment with CH3HgCl in the PBSbuffer.† In this case, the fluorescence saturation is reached with 1.0 equiv. of CH3HgCl (Fig. 1(b)), which can be also explained by the mechanism.
![(a) The time-dependent fluorescence change acquired for a 2 : 1 mixture of probe 1 and HgCl2; inset: a plot of the fluorescence intensity change as a function of the reaction time. (b) The fluorescence intensity change of probe 1 as a function of equiv. of HgCl2 (■) and CH3HgCl ( ), taken after 10 min for each addition. (c) A plot of fluorescence intensity vs. [HgCl2] obtained for a 2 : 1 mixture of 1 and HgCl2 for the range of 0.25–20 ppb [HgCl2], taken after 1 h of each mixing. (d) The fluorescence change after 1 h acquired for a 2 : 1 mixture of probe 1 and various metal ions (Mg2+, Ca2+, Ba2+, Cr2+, Mn2+, Fe3+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+, Pb2+, Ag+ and Hg2+). All measurements were taken with 5.0 μM of probe 1 in PBSbuffer (pH 7.4) containing 5% DMSO (excitation at 480 nm; the intensity was estimated by the peak height at λ = 520 nm).](/image/article/2009/CC/b900380k/b900380k-f1.gif) | ||
Fig. 1 (a) The time-dependent fluorescence change acquired for a 2 : 1 mixture of probe 1 and HgCl2; inset: a plot of the fluorescence intensity change as a function of the reaction time. (b) The fluorescence intensity change of probe 1 as a function of equiv. of HgCl2 (■) and CH3HgCl ( ), taken after 10 min for each addition. (c) A plot of fluorescence intensity vs. [HgCl2] obtained for a 2 : 1 mixture of 1 and HgCl2 for the range of 0.25–20 ppb [HgCl2], taken after 1 h of each mixing. (d) The fluorescence change after 1 h acquired for a 2 : 1 mixture of probe 1 and various metal ions (Mg2+, Ca2+, Ba2+, Cr2+, Mn2+, Fe3+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+, Pb2+, Ag+ and Hg2+). All measurements were taken with 5.0 μM of probe 1 in PBSbuffer (pH 7.4) containing 5% DMSO (excitation at 480 nm; the intensity was estimated by the peak height at λ = 520 nm). ), taken after 10 min for each addition. (c) A plot of fluorescence intensity vs. [HgCl2] obtained for a 2 : 1 mixture of 1 and HgCl2 for the range of 0.25–20 ppb [HgCl2], taken after 1 h of each mixing. (d) The fluorescence change after 1 h acquired for a 2 : 1 mixture of probe 1 and various metal ions (Mg2+, Ca2+, Ba2+, Cr2+, Mn2+, Fe3+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+, Pb2+, Ag+ and Hg2+). All measurements were taken with 5.0 μM of probe 1 in PBSbuffer (pH 7.4) containing 5% DMSO (excitation at 480 nm; the intensity was estimated by the peak height at λ = 520 nm). | ||
Similar fluorescence titrations at different pH conditions (pH 4.0–9.0) were also carried out.† In the absence of HgCl2, probe 1 itself did not show any fluorescence change at pH 4.0, even after 24 h, which indicates that the observed fluorescence change is mainly due to catalysis by mercury species. Comparing the fluorescence recovery time for the cases of HgCl2 and CH3HgCl, the former reacts with probe 1 a little faster than the latter.
When 1 equiv. of HgCl2 was added to probe 1 in the PBSbuffer, the fluorescence saturation took about 1 h (about 70% recovery after 10 min), while it took about 1.5 h in the case of CH3HgCl under the same conditions.† However, it is not necessary to wait for the full recovery of fluorescence for quantification purposes, as we can obtain a linear relationship between the concentration of mercury ions and the fluorescence intensity over an arbitrary time span. A linear relationship between the fluorescence intensity and the concentration of HgCl2 is obtained for a wide concentration range (1.0 × 10−9–1.0 × 10−5 M; 0.2–2000 ppb, a lower concentration part of which is shown in Fig. 1(c)), and a detection limit of below 1 ppb level is obtained.†‡ If an assay of fast and full response is needed, the molar ratio of the probe to the mercury ions can be increased, because an excess amount of the probe gives negligible fluorescence and hence does not affect quantification of the data. This is an advantageous property of probe 1.
Probe 1 is specific towards mercury ions, while other metal ions (Mg2+, Ca2+, Ba2+, Cr2+, Mn2+, Fe3+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+, Pb2+ and Ag+) show essentially no fluorescence change, owing to little hydrolysis of the vinyl ether with these metals (Fig. 1(d)). Also, probe 1 shows full a fluorescence response to the mercury ions, even in the presence of all other metal ions.† Other mercury compounds, such as Hg(OAc)2, gave the same results.
Next, we evaluated the effectiveness of probe 1 for fluorescence monitoring of both CH3HgCl-contaminated cells and a living vertebrate organism, zebrafish. Human lung cancer cells (A549 cells) incubated with probe 1 and CH3HgCl clearly show green fluorescence, indicating that probe 1 can enter cell membranes and react with the organomercury species to form fluorescent 2 (Fig. 2(a)).
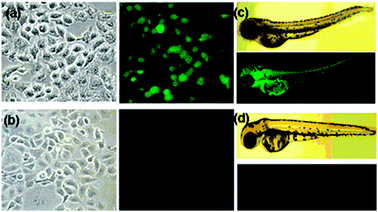 | ||
| Fig. 2 Cells and organisms incubated with 50 mM probe 1 and 100 mM CH3HgCl in 5% CH3CN–water. Images of A549 cells (a) in the presence and (b) absence of CH3HgCl. Images of three-day-old zebrafish (c) in the presence and (d) absence of CH3HgCl. | ||
However, the cells do not exhibit fluorescence in the absence of the external organomercury species (Fig. 2(b)). We explored further whether 1 could be used to monitor organomercury species in living organisms. For this study, a three-day-old zebrafish was exposed to 1 in the presence and absence of CH3HgCl. The results of the fluorescence microscopy analysis show that the organomercury species in zebrafish is fluorescently detected by 1 (Fig. 2(c) and (d)). Encouraged by this result, we analyzed methylmercury accumulation in grown zebrafish with probe 1. Interestingly, the methylmercury accumulated in the eye, heart, fin, gall bladder, and eggs, but not in the brain and liver under the experimental conditions (Fig. 3). These preliminary in vivo studies clearly demonstrate that the probe has potential for studying the accumulation of methylmercury species in other cells and organisms.
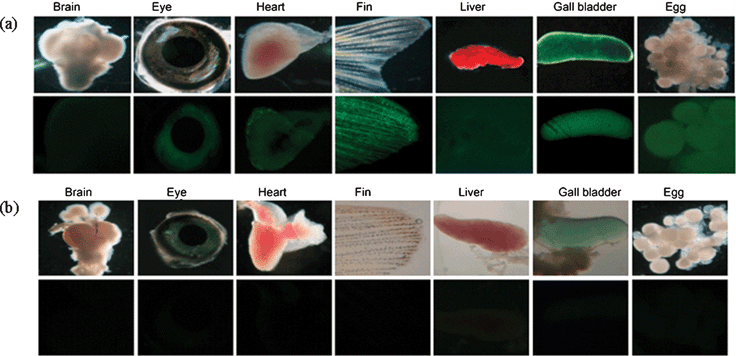 | ||
| Fig. 3 (a) Images of zebrafish organs treated with 200 nM of CH3HgCl and 50 μM of probe 1 (top: microscope images, bottom: fluorescence images). (b) Images of zebrafish organs treated with 50 μM of probe 1 in the absence of external CH3HgCl (top: microscope images, bottom: fluorescence images). | ||
In summary, we have devised a structurally simple yet efficient fluorescent probe for organomercury as well as inorganic mercury ions. The vinyl ether probe 1 thus shows specific response to the mercury species and turn-on fluorescence with high sensitivity, as the vinyl ether undergoes mercury ion-promoted hydrolysis to give the strongly fluorescent 2. With this probe, the fluorescent imaging of mammalian cells and organisms incubated with organomercury species has been demonstrated for the first time.
This work was supported by grants from the EPB center (R11-2008-052-01001) and NRL programs of KOSEF/MEST.
Notes and references
- (a) ATSDR, Toxicological Profile for Mercury, U.S. Department of Health and Human Services, Atlanta, GA, 1999 Search PubMed; (b) ATSDR, ToxProfiles: Mercury, U.S. Department of Health and Human Services, Atlanta, GA, 2005 Search PubMed.
- (a) P. Jitaru and F. Adams, J. Phys. IV France, 2004, 121, 185 Search PubMed; (b) T. W. Clarkson and L. Magos, Off. Rev. Toxicology, 2006, 36, 609 Search PubMed.
- F. Bakir, S. F. Damluji, L. Amin-Zaki, M. Murtadha, A. Khalidi, N. Y. Al-Rawi, S. Tikriti, H. I. Dhahir, T. W. Clarkson, J. C. Smith and R. A. Doherty, Science, 1973, 181, 230 CAS.
- For a recent review, see: (a) E. M. Nolan and S. J. Lippard, Chem. Rev., 2008, 108, 3443 CrossRef CAS; (b) for selected examples, see: A. B. Descalzo, R. Martínez-Máñez, R. Radeglia, K. Rurack and J. Soto, J. Am. Chem. Soc., 2003, 125, 3418 Search PubMed; (c) E. M. Nolan and S. J. Lippard, J. Am. Chem. Soc., 2003, 125, 14270 CrossRef CAS; (d) X. Guo, X. Qian and L. Jia, J. Am. Chem. Soc., 2004, 126, 2272 CrossRef CAS; (e) A. Caballero, R. Martínez, V. Lloveras, I. Ratera, J. Vidal-Gancedo, K. Wurst, A. Tárraga, P. Molina and J. Veciana, J. Am. Chem. Soc., 2005, 127, 15666 CrossRef CAS; (f) S. Yoon, A. E. Albers, A. P. Wong and C. J. Chang, J. Am. Chem. Soc., 2005, 127, 16030 CrossRef CAS; (g) Y.-K. Yang, K.-J. Yook and J. Tae, J. Am. Chem. Soc., 2005, 127, 16760 CrossRef CAS; (h) B. Liu and H. Tian, Chem. Commun., 2005, 3156 RSC; (i) G. Zhang, D. Zhang, S. Yin, X. Yang, Z. Shuai and D. Zhu, Chem. Commun., 2005, 2161 RSC; (j) B. Liu and H. Tian, Chem. Commun., 2005, 3156 RSC; (k) S.-K. Ko, Y.-K. Yang, J. Tae and I. Shin, J. Am. Chem. Soc., 2006, 128, 14150 CrossRef CAS; (l) H. Zheng, Z.-H. Qian, L. Xu, F.-F. Yuan, L.-D. Lan and J.-G. Xu, Org. Lett., 2006, 8, 859 CrossRef CAS; (m) X.-J. Zhu, S.-T. Fu, W.-K. Wong, J.-P. Guo and W.-Y. Wong, Angew. Chem., Int. Ed., 2006, 45, 3150 CrossRef CAS; (n) S. Yoon, E. W. Miller, Q. He, P. H. Do and C. J. Chang, Angew. Chem., Int. Ed., 2007, 46, 6658 CrossRef CAS; (o) H. Yang, Z. Zhou, K. Huang, M. Yu, F. Li, T. Yi and C. Huang, Org. Lett., 2007, 9, 4729 CrossRef CAS; (p) W. Shi and H. Ma, Chem. Commun., 2008, 1856 RSC; (q) X.-Q. Zhan, Z.-H. Qian, H. Zheng, B.-Y. Su, Z. Lan and J.-G. Xu, Chem. Commun., 2008, 1859 RSC.
- A chromogenic chemodosimeter appeared that senses methylmercury species with low sensitivity: O. del Campo, A. Carbayo, J. V. Cuevas, Z. Muñoz, G. García-Herbosa, D. Moreno, E. Ballesteros, S. Basurto, T. Gómez and T. Torroba, Chem. Commun., 2008, 4576 Search PubMed.
- During the publication process of this manuscript, a fluorogenic probe for inorganic mercury ions on the basis of alkyne oxymercuration appeared, which required heating at 90 °C: F. Song, S. Watanabe, P. E. Florencig and K. Koide, J. Am. Chem. Soc., 2008, 130, 16460 Search PubMed.
- (a) F. Song, A. L. Garner and K. Koide, J. Am. Chem. Soc., 2007, 127, 12354 CrossRef; (b) B. A. Sparano, S. P. Shahi and K. Koide, Org. Lett., 2004, 6, 1947 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures for the synthesis of compounds and all the fluorescence analysis data. See DOI: 10.1039/b900380k |
| ‡ The maximum concentration level of mercury ions in drinking water set by the US EPA is 2 ppb. |
| This journal is © The Royal Society of Chemistry 2009 |