Singlet oxygen photosensitisation by the fluorescent probe Singlet Oxygen Sensor Green®†‡
Xavier
Ragàs
,
Ana
Jiménez-Banzo
,
David
Sánchez-García
,
Xavier
Batllori
and
Santi
Nonell
*
Grup d’Enginyeria Molecular, Institut Químic de Sarrià, Universitat Ramon Llull, Via Augusta 390, 08017, Barcelona, Spain. E-mail: santi.nonell@iqs.url.edu; Fax: +34 932 05 62 66; Tel: +34 932 67 20 00
First published on 1st April 2009
Abstract
The fluorescent probe Singlet Oxygen Sensor Green® is able to produce singlet oxygen under exposure to UV or visible radiation.
Singlet oxygen (1O2) is a highly reactive oxygen species (ROS) that can damage biological cell components such as lipids, proteins or nucleic acids. Used intentionally as a deleterious species in photodynamic therapy,1,2 its role as a biological messenger is being increasingly recognized.3–5
Methods for 1O2 detection include EPR spectroscopy using spin traps,6 phosphorescence at 1270 nm7 and chemical trapping.8 The current spread of fluorescence imaging techniques has lead to the development of a number of 1O2fluorescent probes, such as trans-1-(2′-methoxyvinyl)pyrene (MVP),9 dansyl-2,2,5,5-tetramethyl-2,5-dihydro-1H-pyrrole (DanePy),10 or fluorescein-based probes such as DMAX or DPAX.11 Invitrogen/Molecular Probes has recently marketed a highly selective sensor for 1O2 without any appreciable response to hydroxyl radicals or superoxide, under the trade name Singlet Oxygen Sensor Green (SOSG) reagent®.12 While the exact structure of SOSG has not been disclosed, its absorption spectrum resembles that of DMAX and it may therefore be assumed to contain a fluorescein bound to a dimethylanthracene derivative.
SOSG has been successfully applied to the detection of 1O2 in fields as diverse as light-activated plant defence,13 photoinactivation of Staphylococcus aureus bacteria,14 or plasmonic engineering of 1O2 production.15 A recent review compares the benefits of these probes for 1O2 imaging in plants.16
SOSG exhibits weak blue fluorescence peaks at 395 and 416 nm under excitation at 372 and 393 nm. In the presence of 1O2, SOSG emits green fluorescence, with excitation and emission maxima at 504 and 525 nm, respectively.12 This green fluorescence emission was assigned to an endoperoxide generated by the interaction of 1O2 with the anthracene component of SOSG, as observed for other fluorescein–anthracene probes such as DMAX or DPAX.11
In the course of our studies using SOSG as 1O2 probe, we observed the appearance of green fluorescence upon exposure of SOSG to either UV or visible radiation, even in the absence of external 1O2 photosensitisers. This observation has also been reported by other authors.16,17 In the present study, we show that SOSG is able to produce 1O2 under photoirradiation. We also demonstrate that the green fluorescence fades away under UV irradiation as a consequence of SOSG photobleaching mediated by radical species. These results should be taken into account by users of this probe for the design of appropriate controls.
The formation of 1O2 by SOSG has been assessed by time-resolved near-IR phosphorescence detection.18,19 The irradiation of a methanol-d4 SOSG solution at 355 nm produced a clear time-resolved luminescence signal with a maximum intensity at 1275 nm (Fig. 1) and the addition of NaN3 reduced the phosphorescence decay lifetime (see the ESI† ). Likewise, removing oxygen or SOSG from the solution completely eliminated the signal. This set of observations is consistent with the assignment of the emission to 1O2.
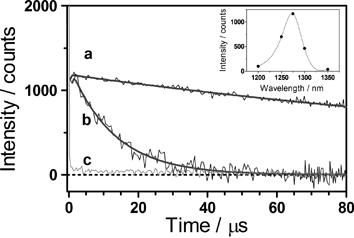 | ||
| Fig. 1 Singlet oxygen phosphorescence photosensitised by SOSG in methanol-d4 The solution was irradiated with 1-ns laser pulses at 355 nm (ca. 1 μJ per pulse, repetition rate 10 kHz) and the luminescence was observed at 1275 nm. (a) Air atmosphere and no sodium azide. (b) Air atmosphere and 0.27 mM sodium azide. (c) Argon atmosphere and no sodium azide. Inset: spectral distribution of the photoluminescence generated by SOSG. | ||
The 1O2 production quantum yield, ΦΔ, was determined by comparing the intensity of the 1O2 signal shown by SOSG to that of optically-matched solutions of reference photosensitisers.18 Using phenalenone and rose bengal as standards (ΦΔref = 0.97 and 0.75, respectively20–22), we obtained ΦΔ values in methanol of 0.03 ± 0.01 and 0.009 ± 0.003 for excitation at 355 and 532 nm, respectively. These quantum yields are modest but not negligible, particularly as SOSG is used as a 1O2 probe. This is particularly important when UV radiation is used or when the photosensitisers under scrutiny show low 1O2 quantum yields, e.g., with the enhanced green fluorescent protein (EGFP).7 Because SOSG is intended for use in aqueous environments, we also measured the quantum yield in 95 : 5 D2O : MeOH mixtures, the residual alcohol being needed to dissolve the probe. In this medium, ΦΔ dropped to 0.006 ± 0.002 at 355 nm and to 0.002 ± 0.001 at 532 nm.
The excitation wavelength dependence of the ΦΔ values indicates that different photochemical processes arise upon excitation of the anthracene or fluorescein moieties. In agreement with this observation, the absorbance and green fluorescence intensity of SOSG changed in a different fashion upon irradiation of either the anthracene moiety (355 nm) or the fluorescein moiety (532 nm). This is depicted in Fig. 2.
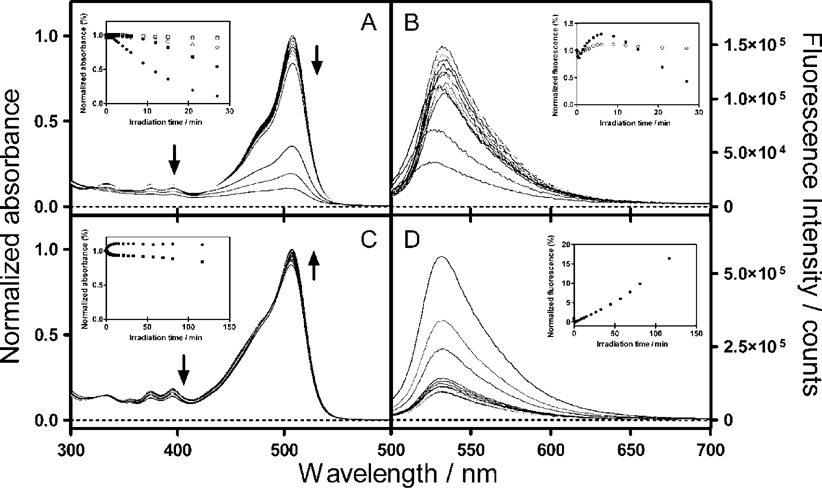 | ||
| Fig. 2 The time course of the absorption (A and C) and fluorescence (B and D) spectra of SOSG upon irradiation in H2O : MeOH (95 : 5) with 10 ns laser pulses at 355 nm (A and B) and 532 nm (C and D) (ca. 20 mJ per pulse, repetition rate 10 Hz). Insets: (A and C) Absorbance at 507 nm (fluorescein moiety, circles) or at 395 nm (anthracene moiety, squares) in the presence (open symbols) and absence (filled symbols) of 100 mM 2-mercaptoethylamine (MEA). (B and D) Area under the fluorescence spectra obtained in the presence (open symbols) and absence (filled symbols) of 100 mM MEA. The samples were excited at 480 nm. | ||
Irradiation at 532 nm induced a transient fluorescence decrease followed by a steady fluorescence rise as the dose was further increased. Interestingly, the absorbance of the fluorescein moiety increased monotonically to a final constant value, while the anthracene moiety was bleached. These results are consistent with a steady production of 1O2 upon irradiation, confirming the time-resolved near-IR luminescence results.
Irradiation at 355 nm induced very different effects: while an increase of the green fluorescence was observed at low irradiation doses, extended irradiation caused it to decrease. At the same time, a fast and extensive bleaching of both the fluorescein and anthracene absorption bands could be observed. The bleaching of the fluorescein moiety could be slowed down by the radical quencher 2-mercaptoethylamine (MEA; 100 mM), but not by 5 mM NaN3, which suggests that it is mediated by radical species but not by 1O2. This observation agrees with an earlier report on the photobleaching of fluorescein 23 and was investigated further by laser flash photolysis. Excitation of SOSG at 355 nm produced a long-lived transient with a maximum absorption at 428 nm, which was insensitive to oxygen, but could be quenched by MEA (Fig. 3). The transient was much weaker when SOSG was irradiated at 532 nm.
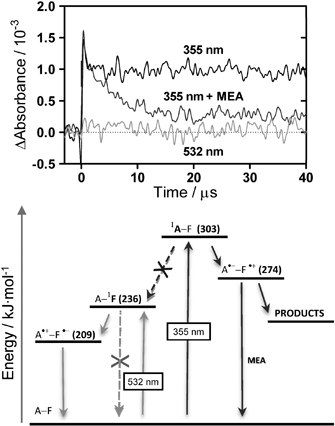 | ||
| Fig. 3 Top: the transient absorption of SOSG in air-saturated H2O : MeOH (95 : 5) solutions as a function of excitation wavelength and added MEA. Observation wavelength: 428 nm. Bottom: energy diagram of the dimethylanthracene–fluorescein photosystem and suggested deactivation pathways following excitation at 355 and 532 nm. | ||
These results can be rationalised using the energy diagram in Fig. 3, under the assumption that the excited-state energy levels and redox potentials of SOSG are close to those of fluorescein and dimethylanthracene.24–26 Irradiation at 355 nm and at 532 nm triggers different photochemical pathways. Thus, irradiation at 532 pumps the fluorescein to its singlet excited state, which is followed by a fast electron transfer from anthracene to fluorescein . As long as the anthracene moiety is intact, this pathway competes efficiently with SOSG fluorescence and the probe is silent. In the presence of 1O2, anthracene is oxidised and this deactivation pathway is no longer available, rendering SOSG fluorescent. However, when intact SOSG is exposed to UV radiation, an additional photochemical pathway becomes possible, namely electron transfer from fluorescein to anthracene, which leads to its irreversible bleaching. The observation of a long-lived transient at 428 nm in the laser flash photolysis experiments is consistent with the formation of the semi-oxidised form of fluorescein in this case.23
In conclusion, the results above show that SOSG is able to act as a 1O2 photosensitiser, particularly under UV radiation. Furthermore, UV irradiation leads to its photobleaching due to the formation of radical species. While SOSG remains a useful 1O2 probe, care must be exercised when using it, particularly in combination with UV sources.
This work was supported by a grant of the Spanish Ministerio de Ciencia e Innovación (CTQ2007-67763-C03-01/BQU). X.R. and A.J.-B. thank the Generalitat de Catalunya (DURSI) and Fons Social Europeu for their predoctoral fellowships.
Notes and references
- D. E. J. G. Dolmans, D. Fukumura and R. K. Jain, Photodynamic therapy for cancer, Nat. Rev. Cancer, 2003, 3, 380–387 CrossRef CAS.
- G. Jori, C. Fabris, M. Soncin, S. Ferro, O. Coppellotti, D. Dei, L. Fantetti, G. Chiti and G. Roncucci, Photodynamic therapy in the treatment of microbial infections: basic principles and perspective applications, Lasers Surg. Med., 2006, 38, 468–481 CrossRef.
- C. Flors and S. Nonell, Light and singlet oxygen in plant defense against pathogens: phototoxic phenalenone phytoalexins, Acc. Chem. Res., 2006, 39, 293–300 CrossRef CAS.
- C. F. Beck, Signaling pathways from the chloroplast to the nucleus, Planta, 2005, 222, 743–756 CrossRef CAS.
- S. Bhattacharjee, Reactive oxygen species and oxidative burst: roles in stress senescence and signal transduction in plants, Curr. Sci., 2005, 89, 1113–1121 CAS.
- E. Hideg, C. Spetea and I. Vass, Singlet oxygen production in thylakoid membranes during photoinhibition as detected by EPR spectroscopy, Photosynth. Res., 1994, 39, 191–199 CrossRef CAS.
- A. Jimenez-Banzo, S. Nonell, J. Hofkens and C. Flors, Singlet oxygen photosensitization by EGFP and its chromophore HBDI, Biophys. J., 2008, 94, 168–172 CAS.
- A. Telfer, S. M. Bishop, D. Phillips and J. Barber, Isolated photosynthetic reaction-center of photosystem-II as a sensitizer for the formation of singlet oxygen-detection and quantum yield determination using a chemical trapping technique, J. Biol. Chem., 1994, 269, 13244–13253 CAS.
- A. Thompson, H. H. Seliger and G. H. Posner, Chemiluminescent probes for singlet oxygen in biological reactions, Methods Enzymol., 1986, 133, 569–584 Search PubMed.
- T. Kalai, E. Hideg, I. Vass and K. Hideg, Double (fluorescent and spin) sensors for detection of reactive oxygen species in the thylakoid membrane, Free Radical Biol. Med., 1998, 24, 649–652 CrossRef CAS.
- K. Tanaka, T. Miura, N. Umezawa, Y. Urano, K. Kikuchi, T. Higuchi and T. Nagano, Rational design of fluorescein-based fluorescence probes. Mechanism-based design of a maximum fluorescence probe for singlet oxygen, J. Am. Chem. Soc., 2001, 123, 2530–2536 CrossRef CAS.
- Molecular Probes Product Information, 2004.
- C. Flors, M. J. Fryer, J. Waring, B. Reeder, U. Bechtold, P. M. Mullineaux, S. Nonell, M. T. Wilson and N. R. Baker, Imaging the production of singlet oxygen in vivo using a new fluorescent sensor, Singlet Oxygen Sensor Green®, J. Exp. Bot., 2006, 57, 1725–1734 CrossRef CAS.
- T. Maisch, J. Baier, B. Franz, M. Maier, M. Landthaler, R. M. Szeimies and W. Bäumler, The role of singlet oxygen and oxygen concentration in photodynamic inactivation of bacteria, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 7223–7228 CrossRef CAS.
- Y. Zhang, K. Aslan, M. J. R. Previte and C. D. Geddes, Plasmonic engineering of singlet oxygen generation, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 1798–1802 CrossRef CAS.
- E. Hideg, A comparative study of fluorescent singlet oxygen probes in plant leaves, Cent. Eur. J. Biol., 2008, 3, 273–284 Search PubMed.
- C. F. Chignell and P. Bilski, personal communication.
- S. Nonell and S. E. Braslavsky, Time-resolved singlet oxygen detection, Methods Enzymol., 2000, 319, 37–49 CAS.
- A. Jiménez-Banzo, X. Ragàs, P. Kapusta and S. Nonell, Time-resolved methods in biophysics. 7. Photon counting vs. analog time-resolved singlet oxygen phosphorescence detection, Photochem. Photobiol. Sci., 2008, 7, 1003–1010 RSC.
- R. Schmidt, C. Tanielian, R. Dunsbach and C. Wolff, Phenalenone, a universal reference compound for the determination of quantum yields of singlet oxygen O2(1Δg) sensitization, J. Photochem. Photobiol., A, 1994, 79, 11–17 CrossRef CAS.
- C. Martí, O. Jürgens, O. Cuenca, M. Casals and S. Nonell, Aromatic ketones as standards for singlet molecular oxygen O2(1Δg) photosensitization. Time-resolved photoacoustic and NIR emission studies, J. Photochem. Photobiol., A, 1996, 97, 11–18 CrossRef CAS.
- F. Wilkinson, W. P. Helman and A. B. Ross, Quantum yields for the photosensitized formation of the lowest electronically excited singlet state of molecular oxygen in solution, J. Phys. Chem. Ref. Data, 1993, 22, 113–262 CAS.
- L. L. Song, C. A. G. O. Varma, J. W. Verhoeven and H. J. Tanke, Influence of the triplet excited state on the photobleaching kinetics of fluorescein in microscopy, Biophys. J., 1996, 70, 2959–2968 CrossRef CAS.
- S. L. Murov, I. Carmichael and G. L. Hug, Ionization Energies, Electron Affinities, and Redox Potentials of Organic Compounds, Handbook of Photochemistry, Marcel Dekker, Inc., New York, 2nd edn, 1993 Search PubMed.
- Y. Urano, M. Kamiya, K. Kanda, T. Ueno, K. Hirose and T. Nagano, Evolution of fluorescein as a platform for finely tunable fluorescence probes, J. Am. Chem. Soc., 2005, 127, 4888–4894 CrossRef CAS.
- H. P. Zhang, Y. L. Zhou, M. H. Zhang, T. Shen, Y. L. Li and D. B. Zhu, Photoinduced interaction between fluorescein ester derivatives and CdS colloid, J. Colloid Interface Sci., 2003, 264, 290–295 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: HPLC, NMR, HRMS and UV-Vis spectra of SOSG; singlet oxygen control experiments. See DOI: 10.1039/b822776d |
| ‡ Dedicated to the memory of Maria Glòria Aguilà |
| This journal is © The Royal Society of Chemistry 2009 |
