Multifunctional bipolar triphenylamine/oxadiazole derivatives: highly efficient blue fluorescence, red phosphorescence host and two-color based white OLEDs†
Youtian
Tao
a,
Qiang
Wang
b,
Yuan
Shang
c,
Chuluo
Yang
*a,
Liang
Ao
a,
Jingui
Qin
a,
Dongge
Ma
*b and
Zhigang
Shuai
*c
aDepartment of Chemistry, Hubei Key Lab on Organic and Polymeric Optoelectronic Materials, Wuhan University, Wuhan 430072, People’s Republic of China. E-mail: clyang@whu.edu.cn
bState Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, People’s Republic of China. E-mail: mdg1014@ciac.jl.cn
cCAS Key Laboratory of Organic Solids, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100080, People’s Republic of China. E-mail: zgshuai@iccas.ac.cn
First published on 20th November 2008
Abstract
Two simple triphenylamine/oxadiazole derivatives were synthesized and fully characterized; their multifunctionality as highly efficient non-doped blue fluorescence, excellent red phosphorescent host and single-doped two-color based white OLEDs has been demonstrated.
Phosphorescent organic light-emitting diodes (PHOLEDs) continue to attract intensive interest because they can approach 100% internal quantum efficiency theoretically. Highly efficient PHOLEDs with emission covering the whole visible region have been reported over the past decade.1–4 However great success has just been achieved in green-light phosphorescence.2,5 Efficient blue PHOLEDs with Commission Internatinale de Eclairage (CIE) y values below 0.2 are very scarce,1a,b and thus there is still a need to develop blue-emitting fluorescent materials with sufficient luminous efficiency and proper chromaticity. On the other hand, the red phosphorescent materials investigated were mostly doped into a 4,4′-bis(N-carbazolyl)biphenyl (CBP) host matrix, which is prone to crystallization when the dopant concentration is too low.4a,6 Furthermore, the driving voltages of red PHOLEDs with the CBP host are usually high because of insufficient and/or unbalanced injection of holes and electrons.7
Recently, bipolar hosts have aroused considerable interest in OLEDs, because they can balance the charge recombination and simplify device structures.8 However, there is a dilemma between the bipolar transporting properties and the band gap of the material, because the electron-donating and electron-withdrawing moieties integrated on bipolar molecules unavoidably lower the band gap of the material due to the intramolecular charge-transfer; while the low triplet energy of the host can cause reverse energy transfer from the guest back to the host, consequently leading to the decreased efficiency of the PHOLEDs. To address this issue, most recently, molecular design focuses on the interruption of π-conjugation between electron-donating and electron-withdrawing moieties by incorporation of steric groups9 and/or meta-linkage10 of the two moieties. In our previous work,11 we firstly reported an ortho-linked carbazole/oxadiazole hybrid molecule as a phosphorescent host, which achieved a maximum current efficiency of 77.9 cd/A for green and 13.6 cd/A for red light.
In this communication, we report a simple combination of commonly used hole-transporter triphenylamine and electron-transporter oxadiazole.12 By introducing the triphenylamine moiety on the o-position relative to the oxadiazole ring of 2,5-diphenyl-1,3,4-oxadiazole, a twisted molecule TPA-o-OXD was acquired, which shows blue-shifted emission, less intramolecular charge-transfer and higher triplet energy levels in comparison with its para-structured analogue TPA-p-OXD. Both compounds show promising bipolar transport properties and highly efficient blue fluorescence. Furthermore, they are proven to be excellent host materials for red electrophosphorescence. Finally, a two-color based white OLED (WOLED) was constructed in a rather simple way by utilization of their dual roles as blue-emitter and host for red phosphorescence. The single-doped and single emissive layer device structure is much simple than the widely reported stacked,13 multi-emissive-layer14 or triple doped15 WOLEDs.
The two compounds TPA-p-OXD (1) and TPA-o-OXD (2) were readily prepared by a Pd(0)-catalyzed Suzuki cross-coupling reaction of 4-(diphenylamino)phenylboronic acid with 2,5-bis(4′-bromophenyl)-1,3,4-oxadiazole or 2,5-bis(2′-bromophenyl)-1,3,4-oxadiazole (Scheme 1). Noticeably, for the steric torsional 2, a high yield of 85% was obtained by using KOH as the base; this is in contrast to a low yield (below 20%) when using Na2CO3 as a base as in the normal Suzuki reaction. The good thermal stability of 1 and 2 is indicated by the high decomposition temperatures (Td, corresponding to 5% weight loss) of 435 °C (1) and 432 °C (2) in the thermogravimetric analysis. The glass-transition temperature (Tg) of 116 °C (1) and 94 °C (2) determined through differential scanning calorimetry (DSC) are much higher than that of CBP (62 °C)16 and comparable to NPB (1,4-bis[(1-naphythylphenyl)amino]biphenyl) (95 °C),17 which is essential for the morphological stability of thin films.18
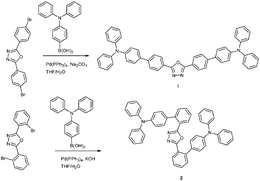 | ||
| Scheme 1 Synthesis of 1 and 2. | ||
Fig. 1 shows the electronic absorption and fluorescence spectra of 1 and 2. The absorption around 300 nm for both 1 and 2 originates from the triphenylamine-centered n–π* transition.9b The absorption at 375 nm for 1 can be attributed to π–π* transition from the electron-donating triphenyamine moiety to the electron-accepting oxadiazole moiety. Notably, the π–π* absorption transition is blue-shifted by 40 nm and remarkably reduced in intensity as only a shoulder for 2, which implies the inhibition of intramolecular charge-transfer. The PL spectrum of the ortho-structured 2 also exhibits a hypsochromic shift of 13 nm with respect to 1 in the solid state. The above phenomena can be attributed to the large space torsion disrupting the π-conjugation between triphenylamine and oxadiazole moieties for 2. The two compounds are blue-emitting with an extraordinarily high quantum yield of 93% for 1 and 74% for 2 in CH2Cl2 solution (Table 1).
| 1 | 2 | |
|---|---|---|
| a T m : melting point. Tg: glass-transition temperature. Td: thermal decomposition temperature. b Absorption maximum, measured in CH2Cl2. c Emission maximum, measured in film. d Measured in CH2Cl2 with an integrating sphere. e Determined from the onset oxidation/reduction potentials of cyclic voltammetry curve in DMF. | ||
| T m /Tg/Td(°C)a | 320/116/435 | 270/94/432 |
| λabs (nm)b | 304, 375 | 309, 335(sh) |
| λem, max (nm)c | 467 | 454 |
| Φ f (%)d | 93 | 74 |
| HOMO/LUMO (eV)e | 5.28/2.60 | 5.25/2.41 |
 | ||
| Fig. 1 Normalized absorption (in CH2Cl2 solution) and PL (film) spectra of 1 and 2 (left) and electroluminescence spectra of the devices (right). | ||
To evaluate the performance of 1 and 2 as blue-emitters, device A with the structure ITO/MoO3 (10 nm)/1,4-bis[(1-naphthylphenyl)amino]biphenyl (NPB, 80 nm)/1 or 2 (20 nm)/2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline (BCP, 10 nm)/Alq3 (30 nm)/LiF (1 nm)/Al (100 nm) was fabricated. NPB and Alq3 were used as the hole- and the electron-transporting materials, respectively, and BCP was used as the hole and exciton blocking layer, while MoO3 and LiF served as the hole- and electron-injecting layers, respectively. All devices are blue-emitting, and representative EL spectra are shown in Fig. 1. Voltage-luminance-current density and current efficiency-current density characteristics for representative devices are shown in Fig. S1 and S2, respectively (ESI†). The device performances are summarized in Table 2. We note that the device derived from 1 emits blue light with a maximum emission of 459 nm and a low energy shoulder at 520 nm; whereas the EL spectrum based on 2 shows an emission peak at 453 nm and no shoulder emission. The suppression of shoulder emission may be contributed to the twisted molecular configuration of 2. This may provide a new strategy to avoid charge-transfer or excimer emission in non-doped blue light-emitting devices. A maximum brightness of 7364 cd/m2 at 13.3 V and a maximum current efficiency of 3.9 cd/A at 3.26 mA/cm2 for 1, and 9441 cd/m2 at 12.9 V and 3.1 cd/A at 0.7 mA/cm2 for 2 were achieved. In addition, all devices display low turn-on voltages in the range 2.7–3.1 V. These performances are comparable with those of non-doped blue-emitting devices reported in literature.9a,17,19
| Device | Compound | V on a | L max b/V | η c c | η ext d | η p e | CIE (x,y)f |
|---|---|---|---|---|---|---|---|
| a Turn-on voltage (V). b Maximum luminance (cd/m2). c Maximum current efficiency (cd/A). d Maximum external quantum efficiency (%). e Maximum power efficiency (lm/W). f Commission International de I’Eclairage coordinates. | |||||||
| A | 1 | 2.8 | 7364, 13.3 | 3.9 | 1.7 | 3.6 | 0.18, 0.31 |
| 2 | 3.3 | 9441, 12.9 | 3.1 | 2.0 | 2.6 | 0.17, 0.19 | |
| B | 1 | 2.7 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 333, 12.9 333, 12.9 |
8.0 | 9.8 | 7.0 | 0.68, 0.32 |
| 2 | 3.1 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 416, 14.9 416, 14.9 |
11.3 | 14.2 | 8.2 | 0.68, 0.32 | |
| C | 1 | 5.3 | 1200, 12.5 | 7.9 | 5.2 | 4.7 | 0.39, 0.31 |
The triplet energies of 1 and 2 were determined to be 2.35 and 2.46 eV, respectively, by the highest-energy vibronic sub-band of the phosphorescence spectra at 77 K, and therefore they may act as appropriate host materials for orange/red-emitting phosphorescent emitters. The OLEDs using 1 or 2 as hosts for red phosphorescent iridium complex bis(1-phenylisoquinolinato-N,C2′) iridium(acetylacetonate) [(piq)2Ir(acac)] were exploited through device B with configurations ITO/MoO3 (10 nm)/NPB (80 nm)/ 1 or 2:(piq)2Ir(acac) (6 wt%, 20 nm)/BCP (10 nm)/Alq3 (30 nm)/LiF (1 nm)/Al (100 nm). Both devices emit red light with a CIE value of (0.68, 0.32). The EL data are summarized in Table 2. A maximum brightness of 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 333 cd/m2 and a maximum current efficiency of 8.0 cd/A was achieved for the 1 based device. The best EL performance was obtained by using 2 as a host, with a maximum brightness of 24
333 cd/m2 and a maximum current efficiency of 8.0 cd/A was achieved for the 1 based device. The best EL performance was obtained by using 2 as a host, with a maximum brightness of 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 416 cd/m2 at 14.9 V, a maximum current efficiency of 11.3 cd/A (corresponding to a maximum external quantum efficiency of 14.2%) and a power efficiency of 8.2 lm/W. When the brightness reaches 1000 cd/m2, the current efficiency is still as high as 9.7 cd/A, which is much better than those CBP hosted devices.20 To the best of our knowledge, these performance data are amongst the best pure red phosphorescent OLEDs with similar CIE coordinates reported in the literature.4a–c
416 cd/m2 at 14.9 V, a maximum current efficiency of 11.3 cd/A (corresponding to a maximum external quantum efficiency of 14.2%) and a power efficiency of 8.2 lm/W. When the brightness reaches 1000 cd/m2, the current efficiency is still as high as 9.7 cd/A, which is much better than those CBP hosted devices.20 To the best of our knowledge, these performance data are amongst the best pure red phosphorescent OLEDs with similar CIE coordinates reported in the literature.4a–c
The better device performance achieved from 2 may be attributed to the enhancement in the localization of charges and higher triplet energy gap as demonstrated by Kakimoto and coworkers and Suet al.9b,c,10a As shown in Fig. 2, the DFT calculated results indicated that 2 has nearly complete separation of the HOMO and LUMO at its hole- and electron-transporting moieties, respectively; whereas 1 exhibits only partial separation of the HOMO and LUMO. The optimized dihedral angles for 2 are larger than those for 1, 55.3° to 36.5° and 23° to 0.3°. The more twisted structure of 2 leads to a significantly higher triplet energy gap for 2 (2.46 eV) than for 1 (2.35 eV).
 | ||
| Fig. 2 DFT calculations of the spatial distributions of the HOMO and LUMO levels for 1 and 2. | ||
By utilizing the dual roles of 1 and 2 as both efficient blue-emitters and excellent hosts for red phosphorescence, white OLEDs were successfully fabricated in a rather simple way with the configuration ITO/NPB (40 nm)/ 1: (piq)2Ir(acac) (0.1 wt%, 20 nm)/BCP (10 nm)/Alq3 (30 nm)/LiF (1 nm)/Al (100 nm) (device C). The EL spectrum at 10 V exhibits the blue fluorescent emission of 1 and the red phosphorescent emission of the iridium complex simultaneously (Fig. 1). A maximum current efficiency of 7.9 cd/A (corresponding to a ηext of 5.2%) at 0.02 mA/m2 and CIE value of (0.39, 0.31) were achieved. The single-doped device structure was much more simple than those stacked, multi-emissive-layer or multi-doped WOLEDs. Further optimization of two-color based WOLEDs by using 1 and 2 is under way.
In summary, we have developed a simple strategy for designing bipolar molecules by the ortho-linkage of electron-donating triphenylamine units and the electron-accepting oxadiazole unit. The twisted molecule showed blue-shifted emission, less intramolecular charge-transfer, and a higher triplet energy level compared to its para-structured analogue. This may provide a new method for solving the dilemma between the bipolar transporting property and band gap of the material. The two compounds were not only used to fabricate non-doped blue-emitting devices with promising performance, but also served as host materials for red phosphorescent guests, achieving performance among the best for pure red phosphorescent OLEDs reported in the literature. Furthermore, we have demonstrated a simple single-doped way to realize two-color based WOLEDs by use of the dual roles of the compounds. We believe the EL performance of WOLEDs will be further improved by carefully optimization of device structures.
We thank the National Natural Science Foundation of China (Project Nos. 50773057 and 20474047) for financial support.
Notes and references
- (a) C.-H. Yang, Y.-M. Cheng, Y. Chi, C.-J. Hsu, F.-C. Fang, K.-T. Wong, P.-T. Chou, C.-H. Chang, M.-H. Tsai and C.-C. Wu, Angew. Chem. Int. Ed., 2007, 46, 2418 CrossRef CAS; (b) C.-F. Chang, Y.-M. Cheng, Y. Chi, Y.-C. Chiu, C.-C. Lin, G.-H. Lee, P.-T. Chou, C.-C. Chen, C.-H. Chang and C.-C. Wu, Angew. Chem. Int. Ed., 2008, 47, 4542 CrossRef CAS; (c) L. Chen, H. You, C. Yang, D. Ma and J. Qin, Chem. Commun., 2007, 1352 RSC.
- (a) M. Ikai, S. Tokito, Y. Sakamoto, T. Suzuki and Y. Taga, Appl. Phys. Lett., 2001, 79, 156 CrossRef CAS; (b) D. M. Kang, J.-W. Kang, J. W. Park, S. O. Jung, S.-H. Lee, H.-D. Park, Y.-H. Kim, S. C. Shin, J.-J. Kim and S.-K. Kwon, Adv. Mater., 2008, 20, 2003 CrossRef CAS; (c) Z. Q. Gao, B. X. Mi, H. L. Tam, K. W. Cheah, C. H. Chen, M. S. Wong, S. T. Lee and C. S. Lee, Adv. Mater., 2008, 20, 774 CrossRef CAS.
- (a) S. Lamansky, P. Djurovich, D. Murphy, F. Abdel-Razzaq, H. E. Lee, C. Adachi, P. E. Buttows, S. R. Forrest and M. E. Thompson, J. Am. Chem. Soc., 2001, 123, 4304 CrossRef CAS; (b) W.-Y. Wong, G.-J. Zhou, X.-M. Yu, H.-S. Kwok and B.-Z. Tang, Adv. Funct. Mater., 2006, 16, 838 CrossRef CAS.
- (a) G. Zhou, W.-Y. Wong, B. Yao, Z. Xie and L. Wang, Angew. Chem. Int. Ed., 2007, 46, 1149 CrossRef CAS; (b) H. Kim, Y. Byun, R. R. Das, K. Choi and P.-S. Ahn, Appl. Phys. Lett., 2007, 91, 093502 CrossRef; (c) J. Huang, T. Watanabe, K. Ueno and Y. Yang, Adv. Mater., 2007, 19, 739 CrossRef CAS.
- (a) S.-J. Su, D. Tanaka, Y.-J. Li, H. Sasabe, T. Takeda and J. Kido, Org. Lett., 2008, 10, 941 CrossRef CAS; (b) S.-J. Su, T. Chiba, T. Takeda and J. Kido, Adv. Mater., 2008, 20, 2125 CrossRef CAS.
- C.-L. Ho, W.-Y. Wong, Z.-Q. Gao, C.-H. Chen, K.-W. Cheah, B. Yao, Z. Xie, Q. Wang, D. Ma, L. Wang, X.-M. Yu, H.-S. Kwok and Z. Lin, Adv. Funct. Mater., 2008, 18, 319 CrossRef CAS.
- S. H. Kim, J. Jang and J. Y. Lee, Appl. Phys. Lett., 2007, 91, 123509 CrossRef.
- (a) Y.-L. Liao, C.-Y. Lin, K.-T. Wong, T.-H. Hou and W.-Y. Hung, Org. Lett., 2007, 9, 4511 CrossRef CAS; (b) K. T. Kamtekar, C. Wang, S. Bettington, A. S. Batsanov, I. F. Perepichka, M. R. Bryce, J. H. Ahn, M. Rabinal and M. C. Petty, J. Mater. Chem., 2006, 16, 3823 RSC.
- (a) M.-Y. Lai, C.-H. Chen, W.-S. Huang, J. T. Lin, T.-H. Ke, L.-Y. Chen, M.-H. Tsai and C.-C. Wu, Angew. Chem. Int. Ed., 2008, 47, 581 CrossRef CAS; (b) Z. Ge, T. Hayakawa, S. Ando, M. Ueda, T. Akiike, H. Miyamoto, T. Kajita and M. Kakimoto, Adv. Funct. Mater., 2008, 18, 584 CrossRef CAS; (c) Z. Ge, T. Hayakawa, S. Ando, M. Ueda, T. Akiike, H. Miyamoto, T. Kajita and M. Kakimoto, Chem. Mater., 2008, 20, 2532 CrossRef CAS.
- (a) S.-J. Su, H. Sasabe, T. i. Takeda and J. Kido, Chem. Mater., 2008, 20, 1691 CrossRef CAS; (b) Z. Ge, T. Hayakawa, S. Ando, M. Ueda, T. Akiike, H. Miyamoto, T. Kajita and M. Kakimoto, Org. Lett., 2008, 10, 421 CrossRef CAS.
- Y. Tao, Q. Wang, C. Yang, Q. Wang, Z. Zhang, T. Zou, J. Qin and D. Ma, Angew. Chem. Int. Ed., 2008, 47, 8104 CrossRef CAS.
- (a) N. Rehmann, C. Ulbricht, A. Köhnen, P. Zacharias, M. C. Gather, D. Hertel, E. Holder, K. Meerholz and U. S. Schubert, Adv. Mater., 2008, 20, 129 CrossRef CAS; (b) X. Yang, D. C. Müller, D. Neher and K. Meerholz, Adv. Mater., 2006, 18, 948 CrossRef CAS; (c) C. Wang, A. S. Batsanov and M. R. Bryce, Chem. Commun., 2004, 578 RSC.
- H. Kanno, N. C. Giebink, Y. Sun and S. R. Forrest, Appl. Phys. Lett., 2006, 89, 023503 CrossRef.
- (a) Y.-S. Park, J.-W. Kang, D. M. Kang, J.-W. Park, Y.-H. Kim, S.-K. Kwon and J.-J. Kim, Adv. Mater., 2008, 20, 1957 CrossRef CAS; (b) Y. Sun, N. C. Giebink, H. Kanno, B. Ma, M. E. Thompson and S. R. Forrest, Nature, 2006, 440, 908 CrossRef CAS.
- B. W. D’Andrade, R. J. Holmes and S. R. Forrest, Adv. Mater., 2004, 16, 624 CrossRef CAS.
- M.-H. Tsai, Y.-H. Hong, C.-H. Chang, H.-C. Su, C. g.-C. Wu, A. Matoliukstyte, J. Simokaitiene, S. Grigalevicius, J. V. Grazulevicius and C.-P. Hsu, Adv. Mater., 2007, 19, 862 CrossRef CAS.
- J. N. Moorthy, P. Venkatakrishnan, D.-F. Huang and T. J. Chow, Chem. Commun., 2008, 2146 RSC.
- F. Santerre, I. Bedja, J. P. Dodelet, Y. Sun, J. Lu, A. S. Hay and M. D’Iorio, Chem. Mater., 2001, 13, 1739 CrossRef CAS.
- (a) Z. Peng, S. Tao, X. Zhang, J. Tang, C. S. Lee and S.-T. Lee, J. Phys. Chem. C, 2008, 112, 2165 CrossRef CAS; (b) Y.-I. Park, J.-H. Son, J.-S. Kang, S.-K. Kim, J.-H. Lee and J.-W. Park, Chem. Commun., 2008, 2143 RSC.
- Y.-J. Su, H.-L. Huang, C.-L. Li, C.-H. Chien, Y. T. Tao, P.-T. Chou, S. Datta and R.-S. Liu, Adv. Mater., 2003, 15, 884 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures, DFT calculation, OLEDs fabrication and characterization. See DOI: 10.1039/b816264f |
| This journal is © The Royal Society of Chemistry 2009 |
