Quantitative SERRS immunoassay for the detection of human PSA†
Ross
Stevenson
a,
Andrew
Ingram
a,
Hing
Leung
b,
Donald C.
McMillan
b and
Duncan
Graham
*a
aCentre for Molecular Nanometrology, WestCHEM, Department of Pure and Applied Chemistry, University of Strathclyde, Glasgow, UK G1 1XL. E-mail: duncan.graham@strath.ac.uk
bDivision of Cancer Sciences and Molecular Pathology, University of Glasgow, Glasgow, UK G11 6NT
First published on 17th March 2009
Abstract
We report the first use of a commonly used ELISA colorimetric substrate as a SERRS marker and show how it can be used for the detection of pg ml−1 levels of human prostate specific antigen (PSA) in clinical samples. The technique is amenable over a wide range of concentrations and lends itself to future multiplexing analysis.
Raman spectroscopy is a spectroscopic technique that gives unique vibrational fingerprints capable of distinguishing structurally similar molecules. The size of Raman signal can be significantly improved by the introduction of a highly scattering neighbouring surface and careful consideration of excitation wavelength leading to excellent limits of detection. Surface enhanced resonance Raman scattering (SERRS) utilises both improvements, and enhancements of up to 1014 can be seen over conventional Raman.1 The potential SERRS offers as a powerful tool for bioanalysis has been attracting great interest recently. With single-molecule detection possible2,3 and potential for multiplexing analysis that outstrips other mainstream spectroscopic techniques,4 the reliability and low-costs allow SERRS to compete with existing analytical techniques over a wide range of applications.
SERRS has been extensively used for DNA detection,5–9 and when using fluorescently-labelled oligonucleotides can demonstrate a lower limit of detection than fluorescence.10Enzyme activity measurements using SERRS have been reported using lipases,11alkaline phosphatase,12 proteases13 and peroxidase.14 In addition there have been numerous methods reported for employing SERRS in immunoassays, such as reporter-labelled nanoparticles conjugated to antibodies,15,16 glass-coated dye-labelled nanoparticles coated to antibodies,17dye-18 or fluorescently-labelled antibodies (with subsequent Ag staining)19 and even label-free formats.20 However, very few methods reporting SERRS as a readout technique for an enzyme linked immunosorbent assay (ELISA) have been reported.
The ELISA is a fundamental technique used by molecular biologists for protein detection and quantification, using the spectroscopic detection of a coloured reagent. The reagents used depend on the choice of antibody conjugate, some of the most common include: 4-methylumbelliferyl phosphate (MUB), 4-nitrophenyl phosphate, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 5-bromo-4-chloro-3′-indolyphosphate p-toluidine (BCIP), 3,3′,5,5′-tetramethylbenzidine (TMB), and o-phenylenediamine. Of the three most commonly used ELISA enzymes (horseradish peroxidase (HRP), alkaline phosphatase, and β-galactosidase), HRP is the most desired antibody conjugate due to it being the smallest and most stable.21 Thus by utilising the sensitivity limits afforded by SERRS coupled with the HRP detection system commonly used in an ELISA assay, significant improvements over the current limits of detection of standard ELISAs may be possible.
There are limited reports on the use of SERRS as a detection technique for ELISA. Dou et al. successfully converted para-diaminobenzene to an azo derivative, using an HRP-conjugate and successfully used this transformation to quantify a mouse-IgG.14 The authors noted, however, several limitations to their technique. For example, the effective measurement range of antigen was very narrow (0.2–2.5 ng ml−1) and the starting material fluoresced strongly at the laser wavelength used, which may interfere with the overall sensitivity of the system. Recently Ruan et al. reported the detection of alkaline phosphatase using SERRS.12 In their system BCIP was transformed into a blue product by the action of alkaline phosphatase. This new chromophore was resonance enhanced using a 633 nm laser source, and possessed a new SERRS-active vibration at 600 cm−1, a useful characteristic peak. The authors demonstrated that this system could be used for single-molecule detection of alkaline phosphatase and suggest its potential use in an immunoassay format. This was subsequently proven by Campbell et al., who successfully used this BCIP–SERRS–ELISA system to detect C-reactive protein at concentrations down to 0.3 ng ml−1.22
This study reports the first use of SERRS as a readout technique in an HRP ELISA using a commercially available substrate, ABTS, as the enzyme-transformable chromogen. We have applied the technique to allow the detection of prostate specific antigen (PSA) in clinical samples. PSA has been described as the most valuable biomarker currently available for the detection of prostate cancer,23 and recent evidence suggests that the currently accepted decisive limit of 4 ng ml−1 of PSA in serum misses a significant number of cancers.24 Here we show how the use of SERRS detection for an ABTS ELISA can allow the detection of pg ml−1 levels of PSA.
To detect the SERRS signal, gold nanoparticles were used and the system excited at 514.5 nm with specific detection of Raman shifts at 1442 cm−1 and 1405 cm−1. Gold nanoparticles offer greater biocompatibility over the silver alternative, they are less likely to precipitate out of solution than silver, and although the ABTS signals could also be obtained from silver nanoparticles, there was no significant enhancement over gold. Fig. 1 shows the spectra obtained with the PSA concentration at 60 ng ml−1 and 0.47 ng ml−1.
A PSA ELISA kit (R&D Systems) was used to obtain a PSA standard calibration. The colorimetric detection of ABTS, calculated as the mean plus two standard deviations from the control sample, had a limit of 500 pg ml−1. As shown in Fig. 2, PSA can be detected across the concentration range by monitoring the SERRS peak heights in triplicate at either 1405 cm−1 or 1442 cm−1.
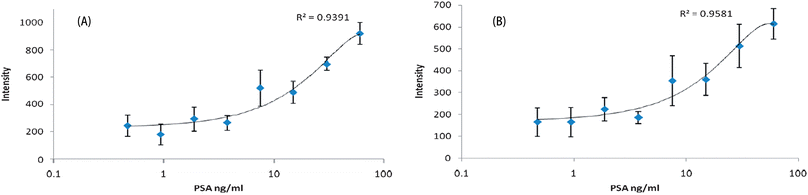 | ||
| Fig. 2 Intensity vs. PSA concentration when monitoring the peak at 1405 cm−1 (A) and 1442 cm−1 (B). Samples were monitored in triplicate and error bars are shown. | ||
To optimise the SERRS response, a time course experiment was carried out as shown in Fig. 3. Simultaneous experiments were carried out where (1) the enzyme acted on the substrate in a gold solution, or (2) the enzyme acted on ABTS in a standard solution before addition to gold immediately prior to detection. Optimum results were achieved by monitoring the SERRS response after 29 minutes. Following common experimental protocol, the ABTS/peroxidase reaction should be quenched by the addition of 1% sodium dodecyl sulfate solution (SDS). The Raman signals in both time-course methods as well as the addition of SDS to the colloid solution had little effect on the SERRS response (see Fig. S1, ESI†).
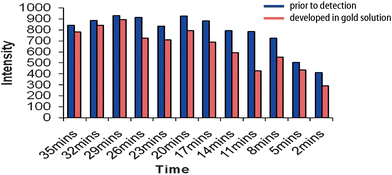 | ||
| Fig. 3 Peak height at 1405 cm−1 was monitored over a time course. The solution was either added to gold immediately prior to detection or was developed in a gold solution. | ||
pH significantly affects the activity of enzymes and most enzyme–antibody conjugates experiments adhere to a tight pH window for optimal response. This appears to be less of a problem for the SERRS detection of ABTS. The peak maxima at 1442 cm−1 was analysed when the reaction had proceeded at specified pH values from 3.5 to 6.5. No significant changes were observed in either the one specific peak (see Fig. S2, ESI†) or across the complete spectrum (data not shown).
To observe whether the ABTS SERRS assay is amenable to real life samples, the assay was used to detect PSA in human serum (Table 1). These samples had previously been used in the diagnosis of prostate cancer. The level of PSA was established and a blind test used to assess the performance of the SERRS assay.
| Sample | SERRS/ng ml−1 | ELISA/ng ml−1 |
|---|---|---|
| A | 16.58 ± 0.36 | 17.32 ± 0.42 |
| B | 15.89 ± 0.55 | 11.61 ± 0.38 |
| C | 101.21 ± 0.124 | 107.98 ± 0.36 |
| D | 42.96 ± 0.78 | 34.21 ± 0.33 |
| Control | 0.97 ± 0.36 | 1.07 ± 0.19 |
The low limit of detection of the coloured product, and accordingly antigen, using SERRS underlines the technique's sensitivity. This new SERRS ELISA offers several distinct advantages over the diaminobenzene HRP SERRS ELISA reported previously:14 namely a lower limit of detection, linearity of response over a wider concentration range and lower fluorescence background for starting materials.
This is the first example of a commercially available HRP substrate, ABTS, being used in a SERRS-based assay. The intensity of Raman peaks has been monitored and we have shown that peak height is directly related to the concentration of enzyme reacted ABTS allowing the detection and quantification of PSA in human serum samples. Results were optimised by careful consideration of optimal pH and time analysis and we have shown that quenching the enzyme reaction has little effect on the Raman response.
Notes and references
- X. Zhang, J. Zhao, A. V. Whitney, J. W. Elam and R. P. Van Duyne, J. Am. Chem. Soc., 2006, 128, 10304–10309 CrossRef CAS.
- S. Nie and S. R. Emory, Science, 1997, 275, 1102–1106 CrossRef CAS.
- K. Kneipp, H. Kneipp, G. Deinum, I. Itzkan, R. R. Dasari and M. S. Feld, Applied Spectroscopy, 1998, 52, 175–178 CrossRef CAS.
- K. Faulds, F. McKenzie, W. E. Smith and D. Graham, Angew. Chem., Int. Ed. Engl., 2007, 46, 1829–1831 CrossRef CAS.
- Y. W. C. Cao, R. C. Jin and C. A. Mirkin, Science, 2002, 297, 1536–1540 CrossRef CAS.
- N. R. Isola, D. L. Stokes and T. Vo-Dinh, Anal. Chem., 1998, 70, 1352–1356 CrossRef CAS.
- R. J. Stokes, A. Macaskill, P. J. Lundahl, W. E. Smith, K. Faulds and D. Graham, Small, 2007, 3, 1593–1601 CrossRef.
- K. Faulds, W. E. Smith and D. Graham, Anal. Chem., 2004, 76, 412–417 CrossRef CAS.
- D. Graham and K. Faulds, Chem. Soc. Rev., 2008, 37, 1042–1051 RSC.
- K. Faulds, R. P. Barbagallo, J. T. Keer, W. E. Smith and D. Graham, Analyst, 2004, 129, 567–568 RSC.
- B. D. Moore, L. Stevenson, A. Watt, S. Flitsch, N. J. Turner, C. Cassidy and D. Graham, Nat. Biotechnol., 2004, 22, 1133–1138 CrossRef CAS.
- C. M. Ruan, W. Wang and B. H. Gu, Anal. Chem., 2006, 78, 3379–3384 CrossRef CAS.
- A. Ingram, L. Byers, K. Faulds, B. D. Moore and D. Graham, J. Am. Chem. Soc., 2008, 130, 11846–11847 CrossRef CAS.
- X. Dou, T. Takama, Y. Yamaguchi, H. Yamamoto and Y. Ozaki, Anal. Chem., 1997, 69, 1492–1495 CrossRef CAS.
- D. S. Grubisha, R. J. Lipert, H. Y. Park, J. Driskell and M. D. Porter, Anal. Chem., 2003, 75, 5936–5943 CrossRef CAS.
- J. Ni, R. J. Lipert, G. B. Dawson and M. D. Porter, Anal. Chem., 1999, 71, 4903–4908 CrossRef CAS.
- S. P. Mulvaney, M. D. Musick, C. D. Keating and M. J. Natan, Langmuir, 2003, 19, 4784–4790 CrossRef.
- T. E. Rohr, T. Cotton, N. Fan and P. J. Tarcha, Anal. Biochem., 1989, 182, 388–398 CAS.
- X. X. Han, L. J. Cai, J. Guo, C. X. Wang, W. D. Ruan, W. Y. Han, W. Q. Xu, B. Zhao and Y. Ozaki, Analytical Chemistry, 2008, 80, 3020–3024 CrossRef CAS.
- X. Dou, Y. Yamaguchi, H. Yamamoto, S. Doi and Y. Ozaki, Journal of Raman Spectroscopy, 1998, 29, 739–742 CrossRef CAS.
- S. S. Deshpande, Enzyme Immunoassays: From Concept to Product Development, Kluwer Academic Publishers, Dordrecht, 1996 Search PubMed.
- F. M. Campbell, A. Ingram, P. Monaghan, J. Cooper, N. Sattar, P. D. Eckersall and D. Graham, Analyst, 2008, 133, 1355–1357 RSC.
- T. Steuber, P. Helo and H. Lilja, World J. Urol., 2007, 25, 111–119 CrossRef.
- I. M. Thompson, D. K. Pauler, P. J. Goodman, C. M. Tangen, M. S. Lucia, H. L. Parnes, L. M. Minasian, L. G. Ford, S. M. Lippman, E. D. Crawford, J. J. Crowley and C. A. Coltman, Jr., New Engl. J. Med., 2004, 350, 2239–2246 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: comparison of ratio gold : ABTS and quenching analysis, and pH analysis. See DOI: 10.1039/b902174d |
| This journal is © The Royal Society of Chemistry 2009 |

