Direct analysis of Stevia leaves for diterpene glycosides by desorption electrospray ionization mass spectrometry†
Ayanna U.
Jackson
a,
Alessandra
Tata
b,
Chunping
Wu
a,
Richard H.
Perry
a,
George
Haas
c,
Leslie
West
c and
R. Graham
Cooks
*a
aDepartment of Chemistry, Purdue University, 560 Oval Drive, West Lafayette, IN 47907, USA. E-mail: cooks@purdue.edu; Fax: +01-765-494-9421; Tel: +01-765-494-5262
b“Sapienza” Universita' di Roma, Dipartimento di Chimica e Tecnologie del Farmaco, Piazzale Aldo Moro 5, 00185 Roma, Italy
cKraft Foods Global Brands LLC, 801 Waukegan Road, Glenview, IL 60025, USA
First published on 5th March 2009
Abstract
The analysis of Stevia leaves has been demonstrated without any sample preparation using desorption electrospray ionization (DESI) mass spectrometry. Direct rapid analysis was achieved using minimal amounts of sample (∼0.15 cm × 0.15 cm leaf fragment). Characteristic constituents of the Stevia plant are observed in both the positive and negative ion modes including a series of diterpene ‘sweet’ glycosides. The presence of the glycosides was confirmed via tandem mass spectrometry analysis using collision-induced dissociation and further supported by exact mass measurements using an LTQ-Orbitrap. The analysis of both untreated and hexane-extracted dry leaves proved that DESI can be successfully used to analyze untreated leaf fragments as identical profiles were obtained from both types of samples. Characterization and semi-quantitative determination of the glycosides was achieved based on the glycoside profile within the full mass spectrum. In addition, the presence of characteristic glycosides in an all-natural commercial Stevia dietary supplement was confirmed. This study provides an example of the application of DESI to direct screening of plant materials, in this case diterpene glycosides.
Introduction
The need for new high-potency sweeteners as substitutes for sucrose to alleviate medical and nutritional concerns has increased in recent years. As a result, new classes of low caloric sweet-tasting compounds are being studied. The leaves of Stevia rebaudiana Bertoni, a perennial shrub of the Asteraceae family, native to certain regions of South America, is one natural material currently being investigated as an alternative to sucrose. It is called the ‘sweet herb of Paraguay’ as the leaves contain a complex mixture of diterpenes, triterpenes, tannins, stigmasterol, volatile oils and eight ‘sweet’ diterpene glycosides.1 These eight glycosides consist of a diterpene backbone with a variety of substituent groups which include glucose, rhamnose, and/or xylose. The ‘sweet’ glycosidic component of the leaves is reported to be ∼50–300 times sweeter than sucrose.1 These glycosides are extracted from the Stevia leaf as all-natural zero caloric sweeteners and are currently in use in some countries.Different extraction procedures and chromatographic methods have been used in order to characterize the glycosides from the Stevia leaves.2–4 Liquid chromatography/mass spectrometry (LC/MS) has been employed to characterize the individual glycosides.5–7 Due to the sensitivity and specificity of the technique, mass spectrometry plays an important role in the detection, differentiation and structural elucidation of the natural products, with tandem mass spectrometry representing an important component of the complex mixture analysis experiment.8–12 While these methods have proven successful in the analysis of the plant material, they can be laborious. Therefore, there is an interest in rapid screening methods for plant materials that require no sample preparation and yet provide specific information regarding the chemical make-up of the material. Desorption electrospray ionization (DESI) mass spectrometry,13–15 when combined with tandem mass spectrometry and exact mass measurements, meets these criteria.
Desorption electrospray ionization mass spectrometry (DESI-MS) is an atmospheric pressure ionization mass spectrometric technique used for the analysis of samples under ordinary ambient conditions.13–15 During the DESI experiment, solvent droplets (nebulized using nitrogen gas and charged through the application of high voltage) impact the sample of interest, typically in the solid phase. The primary droplets produce a thin liquid film on the sample surface, into which the analytes dissolve; they are released from this film in secondary droplets and are transferred through an atmospheric pressure interface into a mass spectrometer for analysis.16 Analysis by DESI requires little or no sample preparation and the entire experiment can be done in times on the order of a few seconds. DESI is a robust sampling and ionization technique as experiments can be tailored to the analyte of interest through optimization of the spray solvent.
The potential advantages of ambient ionization methods such as DESI for the direct examination of targeted analytes in foodstuffs, plants and agricultural materials is evident from previous studies on the qualitative and quantitative determinations of specific compounds in complex matrices.17–23 Particularly cogent examples have involved pharmaceutical preparations;24–28 including exact mass measurements using a prototype Orbitrap mass spectrometer.29 In previous work, DESI has been applied to the direct detection and characterization of alkaloids in plant tissues and there is growing interest in its use in targeted studies of compounds in foodstuffs.30 In this study, DESI-MS experiments have been performed directly on raw, dry, untreated Stevia leaves for qualitative and semi-quantitative characterization of the sweet glycosides in leaf fragments. Using a simple aqueous spray solution, DESI-MSn analysis allowed the ready identification and characterization of the glycosides present in the leaf material. Further validation was achieved using exact mass measurements.
Experimental
Chemicals and standards
The desorption electrospray ionization (DESI) spray solvents (acetonitrile (ACN), methanol (MeOH), chloroform and hexane) were purchased from Sigma-Aldrich (St. Louis, MO) and were made up in 18.2 MΩ water (except chloroform and hexane). The S. rebaudiana leaves were purchased from Herbal Advantage, Inc. (Rogersville, MO). A commercial standard of rebaudioside D was obtained from ChromaDex (Irvine, CA) for optimization of the instrumental parameters. A commercial SteviaPlus® packet (SweetLeaf (Gilbert, AZ)) was analyzed to determine the presence of the various glycosides within a commercial Stevia dietary supplement.Desorption electrospray ionization mass spectrometry analysis
DESI analysis was performed using a Thermo-Fisher Scientific LTQ and an OmniSpray™ ion source from Prosolia, Inc. (Indianapolis, IN). Conventional DESI parameters13,14 were used to record these spectra. Optimization of the tube lens and capillary voltages for the higher mass range was obtained by performing electrosonic spray ionization (ESSI)31 using the rebaudioside D standard (1 µg/mL) in both the positive and negative ion modes. The optimized LTQ instrumental parameters include: a tube lens voltage of 250 V (positive mode); −223 V (negative mode), a capillary voltage of 11 V (positive mode); −14 V (negative mode), a source voltage of 4.5 V; a capillary temperature of 200 °C, automatic gain control (AGC) on, and a solvent flow rate of 3–5 µL/min. Leaf fragments (∼0.5 cm × 1 cm) were mounted onto glass microscope slides (Gold Seal (Portsmouth, NH)) by securing the leaf with tape. Smaller leaf fragments (∼0.15 cm × 0.15 cm) were fixed to glass microscope slides using double-sided tape. For the DESI experiments a spray angle of ∼55° was used with the sample being placed 1–2 mm from the MS inlet. The DESI spray source was rastered across the leaf and where necessary its height above the leaf surface was adjusted to account for the unevenness of the leaf samples. Tandem mass spectrometry experiments were performed directly on the leaf material using an isolation window of 1.5–2 Th (Thomson, Th units of atomic mass/elementary charge) to confirm the presence of the ‘sweet’ glycosides. Typically, the ion signal from the plant material lasted on the order of some tens of seconds and sometimes significantly longer. When a ‘sweet spot’ of the leaf was identified, the signal would last on the order of minutes permitting extensive tandem MS experiments on the various glycosides.The same procedures were used with a Thermo-Fisher Hybrid LTQ-Orbitrap fitted with the same Prosolia, Inc. Omnispray™ ion source. For the analysis of the Stevia leaves, sodium taurocholate (m/z 514.284) was used as the lock-mass for exact mass measurements in the negative ion mode and the tetrapeptide MRFA (m/z 524.265) in the positive ion mode. The lock-mass reagents were added to the DESI spray solvent (MeOH : H2O 20 : 80) at concentrations ∼10–100-fold less than that used for the calibration solution to avoid ion suppression of the Stevia glycosides. The LTQ-Orbitrap instrument parameters include a tube lens voltage of ±65 V, a capillary voltage of ±15 V, a source voltage of 5 V, a capillary temperature of 150 °C, automatic gain control (AGC) on, and a solvent flow rate of 3–5 µL/min. While different instrument parameters were used on the LTQ-Orbitrap, the same glycosidic species were observed in the analysis.
Semi-quantitative analysis
Using a crude form of the standard addition method, semi-quantitative experiments were performed directly on the leaf fragments. Since the leaf matrix does not allow proper mixing of the internal standard, the internal standard (1 mg/mL rebaudioside D) was spotted directly on the leaf fragment and allowed to dry prior to analysis. Blank (no internal standard spotted), 1 µL and 3 µL internal standard spotted sections of the Stevia leaves were analyzed in the negative ion mode since the majority of the compounds were observed best in this polarity. A three-point calibration curve was obtained using the sum of the normalized relative signal intensities of the deprotonated and chloride adduct of the rebaudioside D analyte from the full mass spectra. Each point of the curve was the average of three replicates, each of which represented mass spectra recorded over a range of positions typically for a time on the order of 10 seconds. The ratio of the sum of the normalized relative signal intensities of the deprotonated and chlorinated species of each of the ‘sweet’ glycosides to that of rebaudioside D was used along with the calibration curve to approximate the relative concentration of each of the glycosides. From the total concentration of the targeted glycosides, the glycosidic ratios in the Stevia leaves were estimated.Analysis of commercial product
Analysis of an all-natural commercial Stevia supplement was performed by conventional DESI mass spectrometry. A 3 µL sample of the commercial sample (1 mg/mL made up in MeOH : H2O (1 : 1); 3 µg absolute amount) was spotted onto a porous Teflon substrate (Small Parts, Inc., Miami Lakes, FL) and analyzed using a MeOH : H2O (1 : 1) solvent spray with the same instrumental parameters used for the leaf analysis on the Thermo-Fisher Scientific LTQ.Results
Terpene glycosides in leaf
Raw untreated and hexane-extracted (hexane Soxhlet extraction) leaves were analyzed to determine the applicability of DESI to the analysis of the raw Stevia leaves. The hexane extraction was performed to remove the naturally occurring oil residues associated with some plant materials. A comparison between the raw and hexane-extracted leaves yielded similar mass spectra with respect to the glycosides observed in both the negative and positive ion modes. The hexane-extracted leaves did demonstrate a slightly more intense signal but did not provide any additional advantages. For the analyses of the leaves, typically 2–3 leaf fragments were mounted to the glass slide, so several Stevia leaf fragments could be evaluated. Each sample gave a similar profile of the glycosides with the only significant variation occurring in the overall signal intensity. Experiments were preformed on uninterrupted leaf sections to ensure that the outer leaf surface was being sampled versus internal leaf material that could have been sampled through broken edges. The leaves are ∼140 µm thick, so information on the distribution of material as a function of depth within the leaves was not sought, since the scale is too small to be subjected to DESI analysis. This experiment proved that DESI could be used for the direct analysis of the raw Stevia leaves with no prior sample preparation.The leaf fragments examined ranged in size but were on average ∼0.5 cm × 1 cm. An investigation into the smallest leaf fragment that could be handled easily (∼0.15 cm × 0.15 cm) demonstrated that the glycoside profile could still be readily obtained from the small area. Ultimately, the size of leaf capable of being analyzed is only limited to the area covered by the DESI spray, typically on the order of ∼2 mm × 2 mm (non-imaging applications). However, the leaf fragment must be of feasible size to handle and presumably should not be smaller than ∼0.15 cm × 0.15 cm to avoid the leaf sample from entering the mass spectrometer. All data reported hereon were performed from the larger leaf fragments since they are easier to handle, ∼0.5 cm × 1 cm in size.
One of the advantages of DESI compared to other ambient mass spectrometry ionization methods is the ability to tailor the spray solvent to optimize for specific analytes. For the analysis of the Stevia leaves several spray solvent systems were investigated. They included different ratios of methanol, water, acetonitrile and chloroform, and the addition of sodium chloride to promote simple cation adduct formation and ammonium hydroxide to promote deprotonation in the negative ion mode. Given the solubility of the glycosides, MeOH : H2O (20 : 80) proved to be the optimal solvent system.32 The observed Stevia profiles in the positive and negative ion modes using MeOH : H2O (20 : 80) are illustrated in Fig. 1. Using DESI, one is able to detect all ten reported2–7Stevia glycosides by MS or tandem MS experiments in either the positive or negative ion mode.
 | ||
| Fig. 1 DESI mass spectrum of an unprepared Stevia leaf fragment in (a) negative and (b) positive ion mode using spray solvent MeOH : H2O (20 : 80) and reported without background subtraction. The various glycosides observed are labeled numerically. | ||
Glycoside confirmation through tandem MS analysis
In the positive ion mode, salt adducts were observed readily in the full mass spectrum without the addition of any cationizing reagents to the spray solvent. While trace amounts of inorganic salts are present within the instrument, salts are also present within the leaf. For example, potassium and sodium have been reported to be major inorganic constituents within the leaf at 2.6 and 0.031% (w/w), respectively.32 Therefore, the adducts observed are likely to be a result of the leaf matrix. Confirmation of the glycoside salt adducts was achieved via tandem MS analysis using collision-induced dissociation (CID). Fragments of glucose residues (162 Da) were the primary losses observed in the tandem MS spectra. Full confirmation of all the adduct species was not possible as there is nominal mass overlap between the assignment of sodium and potassium adducts of some of the Stevia glycosides. Therefore, exact mass measurements are needed to further validate the observed Stevia adduct species by determining whether particular ions correspond to the sodium or potassium adducts.DESI analysis in the negative ion mode provided more information pertaining to the glycosides in the leaf. In the negative ion mode, the deprotonated species and chloride adducts of the ‘sweet’ glycosides were observed in the full mass spectrum. The chloride adducts observed in the negative ion mode could result from trace chloride within the mass spectrometer or in the leaf matrix; no additives were added to the spray solvent. The compounds observed included: steviolbioside/rubusoside (isomers), m/z 641 (M − H)− and 677 (M + Cl)−; dulcoside, A m/z 787 (M − H)−; stevioside/rebaudioside B (isomers), m/z 803 (M − H)− and 839 (M + Cl)−; rebaudioside C, m/z 949 (M − H)− and 985 (M + Cl)−; rebaudioside A/E (isomers), m/z 965 (M − H)− and 1001 (M + Cl)−. The chloride adduct assignments were further confirmed through observation of the chloride isotope distribution.
Tandem mass spectrometry experiments were performed to confirm the identity of the various glycosides in the negative ion mode also. The Stevia glycosides were readily confirmed through subsequent glycosidic losses of fragments of 162 Da, as also observed in the positive ion mode. Due to the sensitivity of the tandem MS experiment, glycosides not readily observed in the full mass spectrum could be identified using this method. For instance, rebaudioside D was not readily observed in the full mass spectrum using any of the solvent systems investigated. However, when MS/MS experiments were performed, intense fragmentation of rebaudioside D was observed using the MeOH : H2O (20 : 80) solvent system in both positive and negative ion modes (Supplemental Figure 1†). A summary of the glycosides confirmed by DESI is given in Table 1.
|
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Sweet glycoside | MW (Da) | R1 | R2a | Sweetening potency3 | Concentration in leaf (w/w)4 | Glycoside ratio in leaf4 | Confirmed by MS/MS using DESIb | Approx. glycoside ratio in leaf by DESI |
| a glc = glucose, rham = rhamnose, xyl = xylose. b Compound detected in negative ion mode by conventional DESI analysis. c Overlap due to isomers. d Glycoside ratios calculated together. | ||||||||
| Steviolbioside | 642 | H | glc-glc | 100–125 | <0.4% | 0.3–3% | Y | ∼41%c |
| Rubusoside | 642 | glc | glc | 100–120 | <0.4% | N/A | Y | ∼41%c |
| Stevioside | 804 | glc | glc-glc | 150–300 | 4–14% | 43.1–79.6% | Y | ∼31%c |
| Rebaudioside A | 966 | glc | glc(glc)2 | 250–450 | 2–4% | 7.6–9.9d | Y | ∼9%c |
| Rebaudioside B | 804 | H | glc(glc)2 | 300–350 | <0.4% | 0–0.02% | Y | ∼31%c |
| Rebaudioside C | 950 | glc | glc(rham)(glc) | 120–500 | 1–2% | 0.5–6.0% | Y | ∼6% |
| Rebaudioside D | 1128 | glc-glc | glc(glc)2 | 250–450 | <0.4% | 0–0.4% | Y | ∼4% |
| Rebaudioside E | 966 | glc-glc | glc-glc | 150–300 | <0.4% | 5.6–43.2%d | Y | ∼9%c |
| Rebaudioside F | 936 | glc | glc(xyl)(glc) | N/A | <0.4% | 0.04–0.1% | Y | ∼4% |
| Dulcoside A | 788 | glc | glc-rham | 50–120 | 0.4–0.7% | 0.2–0.4% | Y | ∼5% |
Validation of glycosides through exact mass analysis
Given the complex matrix represented by the leaf, exact mass validation of the glycosides is desirable especially in the positive ion mode. In the course of the tandem MS experiments, the primary fragmentation observed was the loss of glucose residues in both the positive and negative ion modes. Validation through exact mass measurements is needed to confirm the identity of species where there is nominal mass overlap. This was done utilizing the high resolution capabilities of the LTQ-Orbitrap, with exact mass measurements validating the assignments in both ionization modes.Prior to measurements with the LTQ-Orbitrap, the exact mass for each compound was determined as well as the mass of the proposed cation and anion adducts. The DESI-LTQ-Orbitrap data for the Stevia leaves in both the positive and negative ion modes are summarized in Table 2. Using the instrument manufacturer's Xcalibur software, the molecular formula for each Stevia species was confirmed in both the positive and negative ion modes. The error observed for the exact mass measurements was typically less than 3 ppm.
| Sweet glycosides | Molecular formula | Theoretical m/z (Da) | Experimental m/z (Da) | m/z Error (Δ ppm) |
|---|---|---|---|---|
| Steviolbioside/rubusoside | C32H50O13 | 641.31787 (M − H)− | 641.315 (M − H)− | −4.467 |
| 677.29454 (M + Cl)− | 677.295 (M + Cl)− | 0.676 | ||
| 665.31436 (M + Na)+ | Not observed | N/A | ||
| 681.28830 (M + K)+ | 681.288 (M + K)+ | −0.440 | ||
| Stevioside/rebaudioside B | C38H60O18 | 803.37069 (M − H)− | 803.372 (M − H)− | 1.633 |
| 839.34737 (M + Cl)− | 839.349 (M + Cl)− | 1.947 | ||
| 827.36692 (M + Na)+ | 827. 370 (M + Na)+ | 2.942 | ||
| 843.34086 (M + K)+ | 843.341 (M + K)+ | −0.146 | ||
| Rebaudioside A/rebaudioside E | C44H70O23 | 965.42351 (M − H)− | 965.424 (M − H)− | 0.506 |
| 1001.39992 (M + Cl)− | 1001.403 (M + Cl)+ | 2.807 | ||
| 989.41974 (M + Na)+ | Not observed | N/A | ||
| 1005.39368 (M + K)+ | 1005.394 (M + K)+ | 0.342 | ||
| Rebaudioside C | C44H70O22 | 949.42860 (M − H)− | 949.427 (M − H)− | 3.109 |
| 985.40528 (M + Cl)− | 985.409 (M + Cl)− | 3.718 | ||
| 973.42564 (M + Na)+ | Not observed | N/A | ||
| 989.39958 (M + K)+ | 989.397 (M + K)+ | −2.478 | ||
| Rebaudioside F | C43H68O22 | 935.41295 (M − H)− | Not observed | N/A |
| 971.38963 (M + Cl)− | 971.389 (M + Cl)− | −0.643 | ||
| 959.40999 (M + Na)+ | Not observed | N/A | ||
| 975.38393 (M + K)+ | 975.384 (M + K)+ | 0.634 | ||
| Dulcoside A | C38H60O17 | 787.37577 (M − H)− | Not observed | N/A |
| 823.35190 (M + Cl)− | 823.354 (M + Cl)− | 1.881 | ||
| 811.37282 (M + Na)+ | Not observed | N/A | ||
| 827.34678 (M + K)+ | 827.345 (M + K)+ | −1.305 |
The adducts observed in the positive ion mode were typically potassium adducts, as further confirmed through observed isotope ratios. Fig. 2 illustrates the exact mass assignments in the positive ion mode with the inset highlighting the potassium isotope distribution. These data further demonstrate that DESI, in combination with exact mass measurements and tandem MS analyses, is a sensitive and powerful technique applicable to the direct analysis of complex systems.
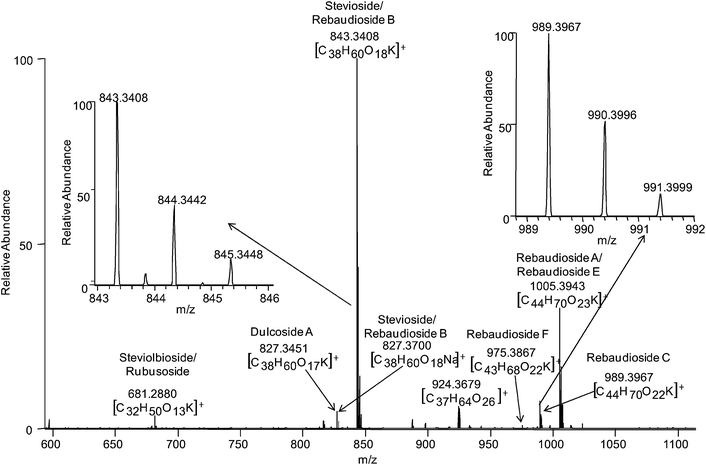 | ||
| Fig. 2 DESI LTQ-Orbitrap mass spectrum of a Stevia leaf fragment in positive ion mode using a spray solvent of MeOH : H2O (20 : 80) 106.7 pmol/µL MRFA (for lock mass analysis). The various glycosides observed are labeled. Insets illustrate the potassium isotope profile of select species. | ||
Exact mass measurements of the LTQ-Orbitrap were extremely valuable in the case of stevioside/rebaudioside B and dulcoside A, isobaric compounds of molecular weight 804 and 788 Da, respectively. In the MS region of m/z 827–829, a potassium isotope signature was observed. However, the base peak of the isotopic group profile did not correspond to the potassium adduct of dulcoside A. Zooming in on this region of the spectrum revealed two ions which were not well resolved, m/z 827.3451 and 827.3696. The sodium adduct of stevioside/rebaudioside B was observed at m/z 827.370 (Δ 2.942 ppm) and this measurement confirmed its formula. The dulcoside A potassium adduct was confirmed by its exact mass 827.345 Da (Δ −1.305 ppm). The resolution of the Orbitrap assisted in confirming the presence of these two isobaric species that could not be properly distinguished using tandem MS experiments on the LTQ. The other regions of the mass spectrum were investigated for this same potential overlap but similar isobars were not observed for the other glycosides.
During the analysis of the leaves, analogs of the sweet glycosides were observed in the negative ion mode. The observed analogs are heavier than the sweet glycosides by 16 Da. While these analogs of the glycosides are not intense in the full mass spectrum, they are of interest as oxidation during DESI has recently been reported.33,34 A brief comparison of the standard compound, rebaudioside D, was made by electrospray ionization (ESI) and conventional DESI. The oxidation products were not readily observed during the ESI analysis but were readily observed by DESI. Since the 16 Da artifact was observed from the rebaudioside D standard as well as the leaf, it may result from glucose (C6H12O6) being oxidized to gluconic acid (C6H12O7) or by the oxidation of the aglycone moiety. This oxidized species was also confirmed via exact mass measurements for select chloride adduct species, namely stevioside/rebaudioside B (C38H60O19Cl−; m/z 855.344 (Δ 2.011 ppm)) and rebaudioside A/E (C44H70O24Cl−; m/z 1017.397 (Δ 2.718 ppm)). In ESI, if redox is observed, it is believed to result from a charge build up at the emitter spray tip as a result of the electrochemical processes that occur during this method of ionization. In DESI, the redox process does not result primarily from electrochemical processes but from a reaction of the analyte with radicals created from discharges of the DESI probe.35 These radicals can be reduced with proper positioning of the DESI probe. Hence, oxidative products are not usually readily observed during the DESI analysis.
Further characterization of Stevia leaf fragments
As previously mentioned, the DESI spray can be tailored for various analytes. An experiment was conducted in which non-polar solvents were added to the spray solvent for the in-situ extraction of the lipid residues. The spray systems investigated included hexane added to chloroform and methanol. Although the use of these spray solvents did not readily yield a profile of the sweet glycosides, adding hexane to the spray solvent provided a fatty acid and lipid profile (m/z 400–700) for both the raw and hexane-extracted leaves in the negative ion mode (Supplemental Figure 2†) possibly corresponding to the cuticle, the thin outer lipidic membrane layer of the leaf. Exact mass and tandem MS studies were not performed using the more hydrophobic solvent. However, the MS profile and m/z values observed correspond to lipids previously observed in the DESI analysis of other biological systems. On this basis, we are confident that the species observed in Supplemental Figure 2 are primarily fatty acids and lipids. This experiment demonstrates that DESI is capable of extracting the sweet glycosides with an optimal spray solvent, MeOH : H2O (20 : 80), from their natural environment which includes their interactions with lipids in the cuticle.Further characterization of the leaves with regards to the location of the sweet glycosides within the leaf was achieved by DESI. The glycosides are known to be highly concentrated within the leaves of the plant and were not observed on the surface of the plant stem, even after direct contact with the Stevia leaves. Seeds, flowers and the roots of the Stevia plant were not available to us for analysis. These results are consistent with the literature where concentration data have only been reported for the leaf and flower components of the plant and not the stem material.32 With regards to the location of the glycosides within the leaf, they appear to be concentrated within the lower cuticle/epidermis layers (underside of the leaf) which is visually duller than the reverse side or upper cuticle/epidermis layers.
Evidence for this was found in the result of experiments performed on the underside and topside of the leaves of both the raw and hexane-extracted leaves. Profiles of the sweet glycosides were only observed during analysis of the undersides of the leaves with varying pigments ranging from deep green to a pale orange. Since the hexane-extracted leaves should contain only traces of oil residues, this analysis suggests that the glycosides are located within the lower epidermis (underside) of the leaf tissue as opposed to the upper epidermis (topside). However, most leaves consist of an upper and lower epidermis separated by a condensed layer of cells, mainly consisting of chloroplast and components needed for photosynthesis, and a less condensed layer of spongy cells into which the pores of the plants open.36 Therefore, the glycosides are predominantly observed on the lower epidermis of the leaves because this is where the pores of the leaves are readily accessed during the DESI experiment.
Semi-quantitative analysis of Stevia glycosides
Depending on the Stevia leaf origin and growth conditions, the relative concentration of the different ‘sweet’ glycosides may vary. Using a form of standard addition (see Experimental section), a semi-quantitative determination of the glycoside distribution was performed using a rebaudioside D standard spotted directly on the leaf. From a three-point calibration curve (R2 = 0.996), the concentration of rebaudioside D within the Stevia leaf fragments was derived. Using the normalized relative intensity ratio of the various glycosides to rebaudioside D in the full mass spectrum, the concentration of each of the glycosides was determined, as was an approximate glycoside ratio within the leaf fragments. Results are reported in Table 1. Given the commercial origin of the Stevia leaves sampled no direct comparison to the literature can be made; however, the numbers calculated are typically within the range or larger than most of the reported values.4 Since DESI is not able to readily distinguish between the isomers of steviolbioside/rebaudioside B, rebaudioside A/rebaudioside E and stevioside/rebaudioside B and other limitations posed by the leaf matrix, these data are only semi-quantitative.Commercial product analysis
DESI analysis of a commercial Stevia supplement was performed to verify that the sweet glycosides could still be readily detected in foodstuff. SweetLeaf SteviaPlus® is an all-natural dietary supplement containing inulin fiber (naturally occurring fructan polysaccharide produced by plants) and the Stevia leaf extract. Analysis of this sample in both the positive and negative ion modes yielded complex spectra with less intense signals for the sweet glycosides than in the leaf spectra (Fig. 3). A dominant oligosaccharide (fructose oligomers) profile due to the fiber was observed in both the positive and negative ion modes. Sodium and potassium adducts were readily observed in the positive ion mode, while the deprotonated species and chloride adducts are present in the negative ion mode of the fiber. The high fiber content of this product is well illustrated within the full mass spectra as is the presence of the Stevia glycosides. Collectively, in both the positive and negative ionization modes, the Stevia glycosides are observed. Therefore, the identification of the glycosides and the fibers present in the commercial dietary supplement demonstrate the powerful utility of the DESI-MS technique in food screening. | ||
| Fig. 3 DESI mass spectrum of commercial SweetLeaf SteviaPlus® product in (a) negative and (b) positive ion mode using MeOH : H2O (1 : 1) spray solvent. The spectra illustrate the presence of inulin fiber (polysaccharides) and select Stevia glycosides which are numerically labeled. Fruc = fructose. | ||
Conclusion
The direct analysis of leaves by DESI has been demonstrated in the analyses of Stevia leaf fragments. From the DESI analyses, profiles of these sweet glycosides which naturally range in concentration within the leaves can be detected with no special additives in the spray solvent. With the use of an ion trap, tandem MS analysis assisted in confirming the identity of the various glycosides except for three pairs of isomers. Exact mass measurements using an LTQ-Orbitrap further confirmed the species observed within 3 ppm mass accuracy. DESI was also used in the semi-quantitative characterization of the relative amounts of the sweet glycosides. Analysis of an all-natural commercial dietary Stevia supplement was also performed demonstrating the presence of the glycosides within a fiber-enriched matrix.The application of DESI to the analysis of plant materials and food products is ideal as the analysis is rapid, qualitative, semi-quantitative (full quantitation dependent upon system) and requires no sample preparation. The DESI experiment can also be extended to the characterization of the distribution of phytochemicals in plant materials with the use of the DESI imaging37 capability or a high-throughput screen to characterize a different plant material. Related experiments on plant hormones have been reported using another ambient ionization method, laser assisted electrospray ionization (LAESI).38,39 Images of the leaf could provide information regarding the specific location and relative concentration and distribution of the analytes within the leaf. On-site field characterization can also be achieved with the use of DESI and a portable miniature mass spectrometer.40,41 This work illustrates the feasibility of DESI for quality control applications within the agricultural and food science fields.
Acknowledgements
Funding at Purdue University was provided by Thermo-Fisher (Grant #1320036658), Alliances for Graduate Education in the Professoriate (AGEP), and the Andrews fellowship (A. U. J.). Additional acknowledgments are extended to Yu Xia and Anthony Costa for useful discussions.References
- J. M. C. Geuns, Phytochemistry, 2003, 64, 913 CrossRef CAS.
- A. S. Dacome, C. C. da Silva, C. E. M. da Costa, J. D. Fontana, J. Adelmann and S. C. da Costa, Process Biochem., 2005, 40, 3587 CrossRef CAS.
- I. C. C. Mantoveli, E. C. Ferretti, M. R. Simeon and C. Ferreira da Silva, Braz. J. Chem. Eng., 2004, 21, 449 Search PubMed.
- J. Pol, B. Hohnova and T. J. Hyotylainen, J. Chromatogr., A, 2007, 1150, 85 CrossRef CAS.
- Y. H. Choi, I. Kirn, K. D. Yoon, S. J. Lee, C. Y. Kirn and K. Yoo, Chromatographia, 2002, 55, 617 CAS.
- J. Pól, E. Varadova, O. Karásek, P. Karásek, M. Roth, K. Benešová, P. Kotlaříková and J. Čáslavský, Anal. Bioanal. Chem., 2007, 388, 1847 CrossRef CAS.
- T. Rajasekaran, A. Ramakrishna, K. U. Sankar, P. Giridhar and G. A. Ravishankar, Food Biotechnology, 2008, 22, 179 CrossRef CAS.
- M. Cui, F. Song, Z. Liu and S. Liu, Rapid Commun. Mass Spectrom., 2001, 15, 586 CrossRef CAS.
- M. Pikulski, A. Aguilar and J. S. Brodbelt, J. Am. Soc. Mass Spectrom., 2007, 18, 422 CrossRef CAS.
- J. Y. Salpin, L. Boutreau, V. Haldys and J. Tortajada, Eur. J. Mass Spectrom., 2001, 7, 321 CrossRef CAS.
- M. Pikulski and J. S. Brodbelt, J. Am. Soc. Mass Spectrom., 2003, 14, 1437 CrossRef CAS.
- B. D. Davis and J. S. Brodbelt, J. Am. Soc. Mass Spectrom., 2004, 15, 1287 CrossRef CAS.
- R. G. Cooks, Z. Ouyang, Z. Takats and J. M. Wiseman, Science, 2006, 311, 1566 CrossRef CAS.
- Z. Takats, J. M. Wiseman and R. G. Cooks, J. Mass Spectrom., 2005, 40, 1261 CrossRef CAS.
- Z. Takats, J. M. Wiseman, B. Gologan and R. G. Cooks, Science, 2004, 306, 471 CrossRef CAS.
- A. B. Costa and R. G. Cooks, Chem. Phys. Lett., 2008, 464, 1–8 CrossRef CAS.
- D. R. Ifa, N. E. Manicke, A. L. Rusine and R. G. Cooks, Rapid Commun. Mass Spectrom., 2008, 22, 503 CrossRef.
- L. Nyadong, S. Late, M. D. Greenc, A. Banga and F. M. Fernendez, J. Am. Soc. Mass Spectrom., 2008, 19, 380–388 CrossRef CAS.
- T. J. Kauppila, J. M. Wiseman, R. A. Ketola, T. Kotiaho, R. G. Cooks and R. Kostiaine, Rapid Commun. Mass Spectrom., 2006, 20, 387 CrossRef CAS.
- Z. Pan, H. Gu, N. Talaty, H. W. Chen, B. E. Hainline, R. G. Cooks and D. Raftery, Anal. Bioanal. Chem., 2005, 77, 6915.
- S. E. Rodriguez-Cruz, Rapid Commun. Mass Spectrom., 2006, 20, 53 CrossRef CAS.
- N. Talaty, Z. Takats and R. G. Cooks, Analyst, 2005, 130, 1624 RSC.
- J. M. Wiseman, S. M. Puolitaival, Z. Takats, R. G. Cooks and R. M. Caprioli, Angew. Chem., Int. Ed., 2005, 44, 7094 CrossRef CAS.
- H. W. Chen, N. N. Talaty, Z. Takats and R. G. Cooks, Anal. Chem., 2005, 77, 6915 CrossRef CAS.
- L. A. Leuthold, J. F. Mandscheff, M. Fathi, C. Giroud, M. Augsburger, E. Varesio and G. Hopfgartner, Rapid Commun. Mass Spectrom., 2006, 20, 103 CrossRef CAS.
- J. P. Williams, V. J. Patel, R. Holland and J. H. Scrivens, Rapid Commun. Mass Spectrom., 2006, 20, 1447 CrossRef CAS.
- J. P. Williams and J. H. Scrivens, Rapid Commun. Mass Spectrom., 2005, 19, 3643 CrossRef CAS.
- D. J. Weston, R. Bateman, I. D. Wilson, T. R. Wood and C. S. Creaser, Anal. Chem., 2005, 77, 7572 CrossRef CAS.
- Q. Hu, N. Talaty, R. J. Noll and R. G. Cooks, Rapid Commun. Mass Spectrom., 2006, 20, 3403 CrossRef CAS.
- N. Talaty, Z. Takats and R. G. Cooks, Analyst, 2005, 130, 1624 RSC.
- Z. Takats, J. M. Wiseman, B. Gologan and R. G. Cooks, Anal. Chem., 2005, 76, 4050.
- Stevia: The Genus Stevia, ed. A. D. KinghornTaylor and Francis,New York, 2002 Search PubMed.
- S. P. Pasilis, V. Kertesz and G. J. Van Berkel, Anal. Chem., 2007, 79, 5956 CrossRef CAS.
- M. Volný, A. Venter, S. A. Smith, M. Pazzi and R. G. Cooks, Analyst, 2008, 133, 525 RSC.
- M. Benassi, C. Wu, M. Nefliu, D. Ifa, M. Volny and R. G. Cooks, Int. J. Mass Spectrom., 2009, 280, 235 Search PubMed.
- D. A. Bird, Plant Science, 2008, 174, 563 CrossRef CAS.
- J. M. Wiseman, D. R. Ifa, Q. Song and R. G. Cooks, Angew. Chem., Int. Ed., 2006, 45, 7188 CrossRef CAS.
- Y. Li, B. Shrestha and A. Vertes, Anal. Chem., 2008, 80, 407–420 CrossRef CAS.
- P. Nemes, A. A. Barton, Y. Li and A. Vertes, Anal. Chem., 2008, 80, 4575–4582 CrossRef CAS.
- A. Keil, N. Talaty, C. Janfelt, R. J. Noll, L. Gao, Z. Ouyang and R. G. Cooks, Anal. Chem., 2007, 79, 7734 CrossRef CAS.
- L. Gao, Q. Song, G. E. Patterson, R. G. Cooks and Z. Ouyang, Anal. Chem., 2006, 78, 5994 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: MS-MS and DESI-MS analysis of Stevia leaf fragments. See DOI: 10.1039/b823511b |
| This journal is © The Royal Society of Chemistry 2009 |

