Electrochemical behavior of lactate dehydrogenase immobilized on “silica sol–gel/nanometre-sized tridecameric aluminium polycation” modified gold electrode and its application†
Jiongjia
Cheng
a,
Deqian
Huang
a,
Jing
Zhang
a,
Wenjing
Yang
a,
Na
Wang
a,
Yongbo
Sun
a,
Keyu
Wang
b,
Xiangyin
Mo
b and
Shuping
Bi
*a
aSchool of Chemistry & Chemical Engineering, State Key Laboratory of Coordination Chemistry of China & Key Laboratory of MOE for Life Science, Nanjing University, Nanjing, 210093, People's Republic of China. E-mail: bisp@nju.edu.cn; Fax: +86 25 83317761; Tel: +86 25 86205840
bMaterials Science Key Laboratory, Nanjing Normal University, Nanjing, 210097, People's Republic of China
First published on 24th March 2009
Abstract
This paper reports the electrochemical behavior of lactate dehydrogenase (LDH) immobilized in the silica sol–gel film on gold electrode after adding nanometre-sized tridecameric aluminium polycation (nano-Al13, also called nanopolynuclear Al13) as a promoter. A pair of surface controlled quasi-reversible cyclic voltammetry peaks with the formal potential (E0′) of 154 mV (vs.SCE) was found in the presence of nano-Al13. A potential application of the nano-Al13-LDH electrode for the determination of resorcinol and p-xylene was also investigated. The experimental results showed that both resorcinol and p-xylene inhibited LDH activity, and the calibration ranges were 5.0 × 10−6–3.0 × 10−4 mol L−1 for resorcinol and 1.0 × 10−6–1.0 × 10−5 mol L−1 for p-xylene, respectively. The nano-Al13-LDH electrode can be anticipated to be applied to environmental toxic assessments.
1. Introduction
The investigations of the electrochemical behavior of enzymes have gained increasing attention in recent years because of their significance in understanding the kinetics of biological redox process.1,2Lactate dehydrogenase (LDH) is one of the most important enzymes and plays a significant role in biological energy metabolism, existing in almost all cells of vertebrates. LDH activity level can be used to indicate several pathological conditions, such as inflammation or lung damage,3 thrombotic thrombocytopenic purpura,4 gastric cancer,5 breast cancer,6etc. In particular, it is considered to be a biomarker and has been applied in the areas of medicine, biology and environment.7 The conversion between pyruvate and lactate catalyzed by LDH can only occur through adding NAD+ (or NADH) as a cofactor in the modifying process or into the experimental solutions.7,8 Thus, the investigation of the electrochemical behavior of LDH would have great importance both in theoretical studies of biological system and in practical applications.Recently, experimental results demonstrated that films with the addition of different nanomaterials could provide a favourable microenvironment for the electron transfer between enzyme and electrode.9 Nanometre-sized tridecameric aluminium polycation (nano-Al13, also called nanopolynuclear Al13), is a kind of manufactured nanomaterial that integrates as linear or ramiform congeries with the general size range from nm to µm10 (see ESI†). It has currently become a research hotspot in environmental science, speciation analysis, geochemistry, biotoxicology and so on, and it is also a new highly efficient inorganic polymer coagulant that has widespread application in water treatment.11
Moreover, nano-Al13 is also a kind of typical nanomaterial that could depolymerize into small oligomeric or monomeric Al species in the reactions,12 which has been proven to be suitable for the studies of interactions between biomolecules and nanomaterials at the molecular level. Therefore, the effect of aluminium species on the biomolecules such as bovine serum albumin has been investigated.13 In the current study, we immobilized LDH on a gold electrode by adding nano-Al13 prepared in our laboratory to investigate its promotion effect on the electrochemical behavior of LDH. The potential application of the nano-Al13-LDH electrode in the determination of environmental pollutants was also studied.
2. Experimental methods
L-LDH (EC 1.1.1.27) (Type III: From Bovine Heart, 549 Unit mg−1) was purchased from Sigma (St Louis, MO, USA). The cation exchange resin of Type 732 (Shanghai Huazhen Sci. & Tech. Co., Ltd., China) and sodium silicate (Na2SiO3·9H2O, Sinopharm Chemical Reagent Co., Ltd., China) was used to prepare the silica sol–gel. A PVA stock solution (0.1%) was prepared by dissolving polyvinyl alcohol (PVA-124, Shanghai Chemical Reagent Plant imported from Japan) in water. Nano-Al13 was prepared from anhydrous aluminium trichloride (AlCl3, Shanghai Meixing Chemical Co., Ltd., China). Resorcinol (0.1 mol L−1) and p-xylene (0.01 mol L−1) stock solutions were prepared by dissolving corresponding resorcinol (Shanghai Baihe Chemical Factory, China) and p-xylene (Nanjing Regent Co., Ltd.) in twice-distilled water, respectively. A phosphate buffer solution (PBS) (0.03 mol L−1) was prepared with Na2HPO4 and KH2PO4. All the chemicals were of analytical grade and were used without further purification. All aqueous solutions were prepared with twice-distilled water.A three-electrode system consisting of a LDH-modified gold working electrode, a saturated calomel reference electrode (SCE) and a platinum counter electrode was used for the electrochemical experiments. A CHI660B Electrochemical Workstation (CH Instruments Inc., USA) was employed for the measurements of cyclic voltammetry (CV), differential pulse voltammetry (DPV), and electrochemical impedance spectroscopy (EIS). DPV measurement was performed with a pulse amplitude of 50 mV and pulse width of 20 ms at a scan rate of 100 mV s−1. 27Al NMR spectra were collected at 130.3 MHz using a DRX-500 superconducting NMR spectrometer (Bruker, Germany). The particle sizes of nano-Al13 was measured by a dynamic light scattering (DLS) method using a Research Goniometer and Laser Light Scattering System (Brookhaven BI200SM, USA).†
The synthesis of nano-Al13 was carried out as described in the literature,14 and the detailed processes are as follows: 25 mL of AlCl3 solution (0.25 mol L−1) was added to a 250 mL double-layer glass beaker that was maintained at a constant temperature of 80 °C with a thermostatic water bath. The solution was titrated with an appropriate volume (according to required OH/Al) of 0.25 mol L−1sodium hydroxide drop by drop (less than 4 mL min−1), cooled and allowed to settle for 24 h. Sulfate crystals were formed by adding 62.5 mL of 0.1 mol L−1 Na2SO4. The crystal was washed twice with distilled water and again twice with 70% ethanol, and finally air dried and stored in a desiccator for further use. The nano-polynuclear aluminium sulfate was characterized by SEM as a tetrahedron structure, and the concentration of nano-Al13 sulfate solution was determined as 0.028 mol L−1 by 27Al NMR under ultrasonic conditions. The particle sizes of K-Al13 aggregates were measured by DLS and found to be in the range of 200–500 nm, similar to the literature reports10c,15 (see ESI†).
The nano-Al13-LDH electrode was prepared according to the following steps: the gold electrode (2 mm in diameter, CH Instruments Inc., USA) was first polished with sand paper and alumina slurry, then washed ultrasonically in ethanol and twice-distilled water, respectively. The cleaned electrode was dipped into the freshly prepared Piranha solution (H2SO4 : 30% H2O2 = 3 : 1) for 2 min and then pretreated electrochemically in 0.5 mol L−1 H2SO4 by cyclic voltammetry. The solution for modification was prepared by mixing silica colloidal sol (the preparation process was as previously described,8a,9b,16), 0.1% PVA solution, nano-Al13 solution, and LDH solution. Finally, the gold electrode was dipped into the mixed solution for 7–12 h at 4 °C in a refrigerator, then dried with nitrogen gas before the electrochemical measurements. All the electrochemical measurements were performed in the PBS buffers at 37 °C. The experimental solutions were deaerated for 15 min with nitrogen gas, and a nitrogen atmosphere was kept over the solutions during the measurements.
3. Results and discussion
3.1 Electrochemical behavior of lactate dehydrogenase after adding nano-Al13 as a promoter
Fig. 1 shows cyclic voltammograms of the bare Au electrode, sol–gel/Al13 electrode, and nano-Al13-LDH electrode in the PBS buffers. A pair of voltammetric peaks with a formal potential (E0′) of 154 mV (vs.SCE) for the nano-Al13-LDH electrode can be observed, reflecting the electrochemical behavior of LDH (Fig. 1, curve c). The current ratio of the cathodic peak current to the anodic one (Ipc/Ipa = 0.87 at 100 mV s−1) and the separation between the anodic and cathodic peak potentials (Epa − Epc = 138 mV at 100 mV s−1) indicated that the electrode process of LDH was a quasi-reversible one on the gold electrode. However, there was only a reduction peak on the sol–gel modified electrode without the addition of nano-Al13,12 the appearance of anodic peak and the positive shifted cathodic peak potential demonstrated that the electron transfer between LDH and electrode was promoted by nano-Al13. On the other hand, the cathodic and anodic peak currents exhibited a linear relationship with the scan rate from 20 to 600 mV s−1, as shown in inset of Fig. 1. This suggested that the electrochemical reaction of LDH immobilized in sol–gel/Al13 film was a typical surface-controlled process, which was in accordance with those of other enzymes.17 The pure nano-Al13 modified electrode, pure LDH modified electrode, and nano-Al13-LDH modified electrode without sol–gel were also characterized by CV and no obvious redox peaks were found (Figures are not shown).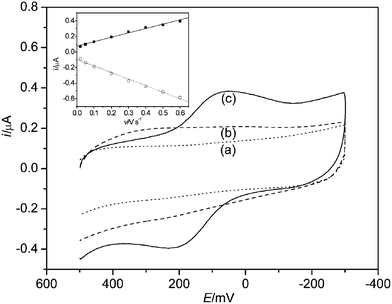 | ||
| Fig. 1 Cyclic voltammograms of (a) bare Au electrode (⋯), (b) sol–gel/PVA/Al13/Au electrode (– – –) and (c) sol–gel/PVA/Al13-LDH/Au electrode (—) at 100 mV s−1 in the PBS buffers (pH 7.0, 37 °C). The concentration of LDH is 625 U mL−1 and the concentration of nano-Al13 is 2.0 mmol L−1. Inset: plot of peak current vs. scan rate. (■) Cathodic peak current and (○) anodic peak current. | ||
3.2 Optimization for preparation of nano-Al13-LDH electrode
The experimental conditions, such as temperature, pH value of the PBS buffers, and the amount of enzyme immobilized on the electrode surface were optimized by investigating the cathodic current response (Fig. 2). A maximum response occurred at 37 °C and pH 7.0. The optimal LDH concentration was found to be 500–800 U mL−1, and 7 hours was chosen as the modifying time. Furthermore, a series of promoters, such as carbon nanotubes, C60, Au nanoparticles, and nano-γ-Al2O3 were chosen to test their influences on the LDH, but no promoting effect was found. It was also found that nano-Al13 had the best promoting behaviour when the concentration was 2.0 mmol L−1. The reproducibility of the nano-Al13-LDH electrode is good, with a relative standard deviation of 4.6% (n = 12) by measuring the cathodic peak current which is similar to the results reported by other groups.18 The nano-Al13-LDH electrode was stored in a sealed container with moisture-conditioning at 4 °C in refrigerator when not in use, and it retained about 91% of the initial current response after a week. | ||
| Fig. 2 Optimization of the experimental parameters for preparation of the nano-Al13-LDH electrode. (A) Effect of temperature on the cathodic responses of sol–gel/PVA/Al13-LDH/Au electrode. (B) Effect of (a) pH value of buffers and (b) concentration of LDH on the cathodic responses of sol–gel/PVA/Al13-LDH/Au electrode. (C) Effect of (a) concentration of nano-Al13 and (b) modifying time on the cathodic responses of sol–gel/PVA/Al13-LDH/Au electrode. Other conditions are the same as Fig. 1. | ||
3.3 The application of nano-Al13-LDH electrode in the determination of pollutants
Application of this electrode in the determination of pollutants could have great significance for environmental assessment, since the threat of persistent organic pollutants has aroused increasing attention nowadays.19 In this experiment, we chose resorcinol and p-xylene, two common environmental pollutants, as examples to study their effect on the electrochemical behavior of LDH. As shown in Fig. 3 and Fig. 4, both resorcinol and p-xylene prohibited the electrochemical response of LDH. The cathodic peak current was negatively linear for the resorcinol concentration from 5.0 × 10−6 to 3.0 × 10−4 mol L−1, and the linear equation was I(µA) = 0.138 − 0.410C (mmol L−1) with a correlation coefficient of 0.9957. On the other hand, the linear response range of p-xylene was from 1.0 × 10−6 to 1.0 × 10−5 mol L−1, and the linear calibration equation was I(µA) = 0.564 − 0.039C (µmol L−1) with a correlation coefficient of 0.9951. It can be concluded that the nano-Al13-LDH electrode might be applied to determine the concentration of pollutants and also could be further developed as a biomarker to give some environmental toxic assessment.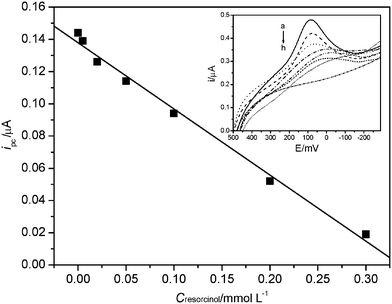 | ||
| Fig. 3 The calibration curve based on the effect of resorcinol on the cathodic peak current of nano-Al13-LDH electrode. Inset: the cathodic part of cyclic voltammograms of sol–gel/PVA/Al13-LDH/Au electrode (curves a–g) and bare sol–gel/PVA/Al13/Au electrode (curve h) at 100 mV s−1 in 0.03 mol L−1 Na2HPO4-KH2PO4 (pH 7.0, 37 °C) in the presence of resorcinol. Different concentrations of resorcinol (mmol L−1): (a) 0, (b) 0.005, (c) 0.02, (d) 0.05, (e) 0.1, (f) 0.2 and (g) 0.3. | ||
 | ||
| Fig. 4 The calibration curve based on the effect of p-xylene on the cathodic peak current of nano-Al13-LDH electrode. Inset: Differential pulse voltammograms of the sol–gel/PVA/Al13-LDH/Au electrode (curves a–f) and bare sol–gel/PVA/Al13/Au electrode (curve g) at 100 mV s−1 in 0.03 mol L−1 Na2HPO4-KH2PO4 (pH 7.0, 37 °C) in the presence of p-xylene. Different concentrations of p-xylene (µmol L−1): (a) 0, (b) 1.0, (c) 2.0, (d) 5.0, (e) 8.0 and (f) 10. | ||
4. Conclusions
In summary, the nano-Al13-LDH electrode shows a good promotion effect towards the electrochemical behavior of LDH, and a pair of well-defined redox peaks was obtained under the optimal conditions of physiological situations (pH 7.0, 37 °C). The promoting mechanism for the nano-K-Al13 for LDH electrochemistry is interesting, but now it is unclear and deserves further studies. Due to the specific configuration of nano-K-Al13, and considering that LDH is composed of units of polypeptides which may have some electroactive amino acids residues, we supposed that the electrochemical behavior of nano-Al13-LDH electrode may be due to the redox reaction of some electroactive amino acids.20 This nano-Al13-LDH electrode can be applied to the determination of environmental pollutants and could be anticipated to provide an efficient strategy for further studies on the electrochemical behavior of enzymes and other practical purposes.Acknowledgements
This project is supported by the NSFC (2077703 & NFFTBS–J0630425), NSFJ (BK2005209), grants from MOE for Ph.D. Program (20050284030), State Key Laboratory of Electroanalysis of China in Changchun Applied Chemistry Institute (2008008), and Analytical Center of Nanjing University.References
- (a) Q. Liu, X. B. Lu, J. Li, X. Yao and J. H. Li, Biosens. Bioelectron., 2007, 22, 3203 CrossRef CAS; (b) Y. H. Wu and S. S. Hu, Microchim. Acta, 2007, 159, 1 CrossRef CAS; (c) Y. Xiao, F. Patolsky, E. Katz, J. F. Hainfeld and I. Willner, Science, 2003, 299, 1877 CrossRef CAS; (d) T. M. Simandi, L. I. Simandi, M. Gyor, A. Rockenbauer and A. Gomory, Dalton Trans., 2004, 1056 RSC; (e) Y. Zheng and X. Q. Lin, Chin. J. Anal. Chem., 2008, 36, 604 CAS; (f) B. G. Jiang, L. Zhang and S. D. Fan, Spectrosc. Spectral Anal., 2008, 28, 609 CAS; (g) P. Steinrucke, U. Aldinger, O. Hill, A. Hillisch, R. Basch and S. Diekmann, Anal. Biochem., 2000, 286, 26 CrossRef CAS.
- (a) Q. L. Wang, G. X. Lu and B. J. Yang, Langmuir, 2004, 20, 1342 CrossRef CAS; (b) H. M. Marques, Dalton Trans., 2007, 4371 RSC; (c) M. S. El-Deab and T. Ohsaka, Electrochem. Commun., 2007, 9, 651 CrossRef CAS; (d) K. Murata, N. Nakamura and H. Ohno, Electroanalysis, 2007, 19, 530 CrossRef CAS.
- M. Drent, N. A. M. Cobben, R. F. Henderson, E. F. M. Wouters and M. Van Dieijen-Visser, Eur. Respir. J., 1996, 9, 1736 CrossRef CAS.
- J. A. Cohen, M. E. Brecher and N. Bandarenko, J. Clin. Apheresis, 1998, 13, 16 CrossRef CAS.
- Y. Kolev, H. Uetake, Y. Takagi and K. Sugihara, Ann. Surg. Oncol., 2008, 15, 2336 Search PubMed.
- R. Coleman, J. Brown and R. Cook, Cancer Treat. Rev., 2008, 34, S78.
- (a) J. E. Basner and S. D. Schwartz, J. Am. Chem. Soc., 2005, 127, 13822 CrossRef CAS; (b) A. D. Ellington and J. J. Bull, Science, 2005, 310, 454 CrossRef CAS.
- (a) O. A. Raitman, E. Katz, A. F. Buckmann and I. Willner, J. Am. Chem. Soc., 2002, 124, 6487 CrossRef CAS; (b) J. W. Di, J. J. Cheng, Q. Xu, H. E. Zheng, J. Y. Zhuang, Y. B. Sun, K. Y. Wang, X. Y. Mo and S. P. Bi, Biosens. Bioelectron., 2007, 23, 682 CrossRef CAS; (c) K. A. Yao, N. Wang, J. Y. Zhuang, Z. B. Yang, H. Y. Ni, Q. Xu, C. Sun and S. P. Bi, Talanta, 2007, 73, 529 CrossRef CAS.
- (a) X. L. Luo, A. J. Killard and M. R. Smyth, Electroanalysis, 2006, 18, 1131 CrossRef CAS; (b) J. W. Di, M. Zhang, K. A. Yao and S. P. Bi, Biosens. Bioelectron., 2006, 22, 247 CrossRef CAS; (c) Q. W. Li, G. A. Luo and J. Feng, Electroanalysis, 2001, 13, 359 CrossRef CAS.
- (a) H. X. Tang and Z. K. Luan, Environ. Chem., 1997, 16, 497 Search PubMed; (b) S. P. Bi, C. Y. Wang, Q. Cao and C. H. Zhang, Coord. Chem. Rev., 2004, 248, 441 CrossRef CAS; (c) H. Z. Zhao, Z. K. Luan, Y. B. Su and S. G. Wang, Chem. J. Chin. Univ., 2002, 23, 751 Search PubMed.
- (a) B. Y. Gao, H. H. Hahn and E. Hoffmann, Water Res., 2002, 36, 3573 CrossRef CAS; (b) J. Liu, F. H. Zhao and J. Q. Liu, Adv. Earth Sci., 2007, 22, 305 Search PubMed; (c) J. W. Akitt, J. M. Elders, X. L. R. Fontaine and A. K. Kundu, J. Chem. Soc., Dalton Trans., 1989, 1897 RSC; (d) A. Drljaca, M. J. Hardie and C. L. Raston, J. Chem. Soc., Dalton Trans., 1999, 3639 RSC.
- (a) W. H. Casey, Chem. Rev., 2006, 106, 1 CrossRef CAS; (b) O. Deschaume, A. Fournier, K. L. Shafran and C. C. Perry, New J. Chem., 2008, 32, 1346 RSC; (c) A. C. Fournier, K. L. Shafran and C. C. Perry, Anal. Chim. Acta, 2008, 60, 61 CrossRef; (d) Z. S. Qian, H. Feng, W. J. Yang, Q. Miao, L. N. He and S. P. Bi, Chem. Commun., 2008, 3930 RSC; (e) Z. S. Qian, H. Feng, W. J. Yang and S. P. Bi, J. Am. Chem. Soc., 2008, 130, 14402 CrossRef CAS; (f) N. Wang, D. Q. Huang, J. Zhang, J. J. Cheng, T. Yu, H. Q. Zhang and S. P. Bi, J. Phys. Chem. C, 2008, 112, 18034 CrossRef CAS; (g) Z. S. Qian, H. Feng, Z. J. Zhang, W. J. Yang, J. Jin, Q. Miao, L. N. He and S. P. Bi, Dalton Trans., 2009, 521 RSC; (h) Z. S. Qian, H. Feng, W. J. Yang, Z. J. Zhang, Y. J. Wang and S. P. Bi, Dalton Trans., 2009, 1554 RSC; (i) K. L. Shafran and C. C. Perry, Dalton Trans., 2005, 2098 RSC.
- O. Deschaume, K. L. Shafran and C. C. Perry, Langmuir, 2006, 22, 10078 CrossRef CAS.
- (a) M. B. Luo, C. Y. Wang, S. J. Liu and S. P. Bi, Chin. J. Inorg. Chem., 2004, 20, 69 CAS; (b) B. Lothenbach, G. Furrer and R. Schulin, Environ. Sci. Technol., 1997, 311, 452; (c) G. Furrer, C. Ludwig and P. W. Schindler, J. Colloid Interface Sci., 1992, 149, 56 CrossRef CAS; (d) E. Molis, F. Thomas, J. Y. Bottero, O. Barres and A. Masion, Langmuir, 1996, 12, 3195 CrossRef CAS.
- (a) H. J. Liu, J. H. Qu, S. J. Zhang and C. Z. Hu, Sci. in China (B), 2002, 45, 515 Search PubMed; (b) Y. B. Chu, B. Y. Gao, Q. Y. Yue, Y. Wang, Y. Z. Liu and C. Y. Kong, Environ. Sci., 2004, 25, 75 Search PubMed.
- J. W. Di, S. P. Bi and M. Zhang, Biosens. Bioelectron., 2004, 19, 1479 CrossRef CAS.
- (a) G. C. Zhao, Z. Z. Yin, L. Zhang and X. W. Wei, Electrochem. Commun., 2005, 7, 256 CrossRef CAS; (b) B. D. Fleming, Y. Tian, S. G. Bell, L. Wong, V. Urlacher and H. A. O. Hill, Eur. J. Biochem., 2003, 270, 4082 CrossRef CAS; (c) X. Jiang, L. Zhang and S. J. Dong, Electrochem. Commun., 2006, 8, 1137 CrossRef CAS.
- (a) J. X. Wang, M. X. Li, Z. J. Shi, N. Q. Li and Z. N. Gu, Anal. Chem., 2002, 74, 1993 CrossRef CAS; (b) L. Wang, J. X. Wang and F. M. Zhou, Electroanalysis, 2004, 16, 627 CrossRef CAS; (c) H. M. Zhang and N. Q. Li, Bioelectrochem., 2000, 53, 97.
- (a) S. K. Ritter, Chem. Eng. News, 2007, 85, 11; (b) J. Turney, Nature, 2006, 444, 819 CrossRef CAS; (c) D. L. Downie, Science, 2003, 300, 901 CAS.
- (a) F. Huerta, F. Morauon, F. Cases, A. Rodes, J. L. Vazquez and A. Aldaz, J. Electroanal. Chem., 1997, 421, 179 CrossRef CAS; (b) R. P. Deo, N. S. Lawrence and J. Wang, Analyst, 2004, 129, 1076 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: summary of different K-Al13 nano-size distributions for different groups. See DOI: 10.1039/b823096j |
| This journal is © The Royal Society of Chemistry 2009 |
