DOI: 10.1039/B820259C
(Highlight)
Lab Chip, 2009, 9, 15-16
Research Highlights
First published on 21st November 2008
Nanodeposition by means of a nanofountain probe
The positioning of single or few molecules at defined positions on a surface is an intriguing challenge with many applications in biology and life sciences, e.g. for biosensing and screening applications. Submicrometer-sized biomolecule arrays can be achieved by several techniques including dip-pen nanolithography, electrospray systems and pipette-type devices. Horacio D. Espinosa and co-workers have recently developed another smart method that is useful for directly patterning a variety of molecules.1 Because the molecules are deposited onto the surface in solution, the method is particularly useful for deposition of proteins, whose function can be highly sensitive to the environment. The “heart” of the novel device is a nanofountain atomic force microscope probe (Fig. 1). This probe tip consists of a pyramidal core tip with a radius of ∼200 nm, surrounded by a volcano-like shell. Fluids are supplied towards the cantilevered nanofountain probe (NFP) via microfluidic channels. Each device contains four fluid reservoirs, each delivering fluids to six nanofountain probes. In order to achieve a reliable and continuous transport of protein solution through the NFP, an electrical field is applied, while the NFP transcribed the desired pattern as guided by the AFM in contact mode. When the NFP reservoir is positively biased, proteins are transported and deposited on the substrate surface. Dots and lines of molecular patterns with submicrometer diameters can be generated with resolution that depended on the magnitude of the applied voltage, dwell time and writing speed, which is demonstrated for positively charged proteins (Immunoglobulin G), as well as for negatively charged biotinylated BSA (bovine serum albumin). The experimental work is supported by theoretical considerations to explain the underlying mechanisms that reveal the dependence of pattern resolution by the tip sharpness rather than by the overall probe aperture. Hence, the NFP approach combines the strength of high-resolution dip-pen nanolithography with the advantage of pipette-based methods that facilitates continuous sample feeding. Moreover, it is readily useable for 1D and 2D probe arrays. | ||
| Fig. 1 Nanodeposition of proteins by means of nanofountain probes. (A) Design of the microfluidic component of the device. (B) SEM image of the end of a single cantilever showing the core tip and the surrounding shell. (C) and (D) Arrays of IgG and of biotin–BSA, respectively, patterned on a substrate. (E) Parallel lines of biotin–BSA deposited on a surface. (Reprinted with permission from O. Y. Loh et al.1 Copyright 2008 National Academy of Sciences, USA). | ||
Microfluidics on paper
The great potential of microfluidic devices for point-of-care diagnostics has been widely demonstrated in recent years. However, for frequent use, e.g. for daily health (or disease) monitoring, the costs for the device and the chemicals required for the assays have to be reasonably low. Among the possible materials for a cheap device, cellulose-based paper is one suitable choice. It has long since been used as a cheap chromatographic substrate, and in recent studies, it has been shown to be useful for open-channel microfluidics as well, in which the microchannels on the paper have been defined by means of photoresist patterns or penetration of polydimethylsiloxane into the paper. However, since paper is a non-uniform porous and flexible material, the transfer of microfluidic channels into or onto the paper, respectively, requires specific techniques that would not affect the flexibility or topology of the paper. Wei Shen and co-workers from the Australian Pulp and Paper Institute in Victoria have reported a method that meets these needs to define microfluidic channels on a filter paper surface.2 The paper was first homogeneously hydrophobised by dipping it into a solution of alkyl ketene dimer dissolved in n-heptane. After removal of the organic solvent and heating, the paper had a strong hydrophobic surface. The paper was afterwards sandwiched between metal masks that have the desired pattern and placed in a vacuum plasma reactor for a few seconds to create the hydrophilic pattern. A droplet of water can be guided along these hydrophilic pathways by capillary penetration. The researchers suggest several functional elements such as a filter and a reactor with two dosing sites. Unlike previously reported procedures, the paper flexibility is retained and thus, bending of paper would not damage the hydrophilic patterns. Indeed, folding of paper could be part of the device design.Microfluidics integrated with cryogenic transmission electron microscopy
Surfactants and lipid molecules form a rich variety of nano- and microstructures in solution. In previous studies, the formation process could be explained by a structural transition from initially formed micelles to vesicles through disk-like intermediate states. Anubhav Tripathi and co-workers from the University of Rhode Island have recently proposed an alternative pathway, which they could observe by means of microfluidics combined with cryogenic transmission electron microscopy (cryo-TEM).3 They employed a microfluidic chip to form micelles from a specific amphiphilic system (solutions of cetyltrimethylammonium bromide, CTAB, and dodecylbenzene sulfonic acid, HDBS). Flow rates and hence, diffusive transport and residence time on structure formation could be controlled carefully. The important development is the integration of the microchip with a controlled environment vitrification system (Fig. 2). The solution is ejected on a holey carbon grid and subsequently plunged into liquid ethane to complete vitrification. The formed structures can be directly imaged in a TEM. The system facilitates the observation of intermediate formation states. The authors could disclose a formation route of vesicles, in which first, micelles aggregate to long tubules, which became unstable and break up into vesicles. This pathway can be understood on basis of simple energy arguments.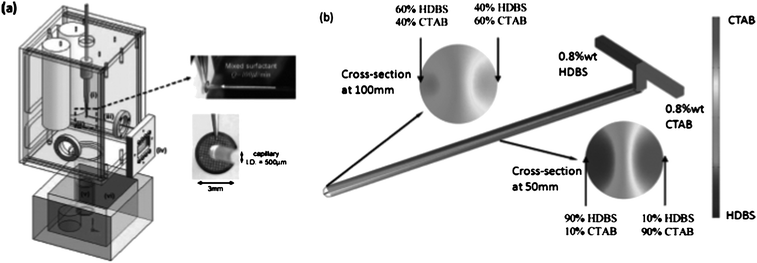 | ||
| Fig. 2 Microfluidics integrated with a vitrification system to image fast self-assembly processes. (a) Scheme of the device that connects the microfluidic chip with a fast cooling unit (liquid ethane) via a holey carbon grid. Afterwards, the sample is imaged in a cryo-TEM. (b) Visualisation of concentration profiles of the amphiphilic compounds used in these experiments (HDBS: dodecylbenzene sulfonic acid and CTAB: cetyltrimethylammonium bromide). (Reprinted with permission from J. Lee et al.3 Copyright 2008 American Chemical Society). | ||
The developed system is very versatile to study formation of nanostructures composed of other materials as well, and hence could improve the understanding of self-assembly processes.
Petra S. Dittrich
ETH Zürich, Switzerland
dittrich@org.chem.ethz.ch
References
- O. Y. Loh, A. M. Ho, J. E. Rim, P. Kohli, N. A. Patankar and H. D. Espinosa, Electric field-induced direct delivery of proteins by a nanofountain probe, Proc. Natl. Acad. Sci. U. S. A, 2008, 105, 16438–16443 CrossRef CAS.
- X. Li, J. Tian, T. Nguyen and W. Shen, Paper-based Microfluidic Devices by Plasma Treatment, Anal. Chem., 2008 DOI:10.1021/ac801729t.
- J. Lee, A. K. Jha, A. Bose and A. Tripathi, Imaging New Transient Nanostructures Using a Microfluidic Chip Integrated with a Controlled Environment Vitrification System for Cryogenic Transmission Electron Microscopy, Langmuir, 2008, 24, 12738–12741 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2009 |
