Lignans by photo-oxidation of propenyl phenols
Marina
DellaGreca
*,
Maria R.
Iesce
*,
Lucio
Previtera
,
Raffaella
Purcaro
,
Maria
Rubino
and
Armando
Zarrelli
Dipartimento di Chimica Organica e Biochimica, Complesso Universitario Monte Sant'Angelo, Via Cinthia 4, I-80126 Naples, Italy. E-mail: dellagre@unina.it; iesce@unina.it; Fax: +39 081 674393; Tel: +39 081 674162
First published on 26th October 2007
Abstract
Dimeric cyclic or acyclic lignan derivatives have been obtained from isoeugenol under light induced oxidations (mainly by MB-sensitized photooxygenation in water/CH3CN or di-tert-butyl peroxide photo-oxidation). Coniferyl alcohol gives lignans only under peroxide-mediated photo-oxidation while ferulic acid was unreactive under both conditions.
Introduction
Lignans have attracted a great deal of interest over the years due to their wide occurrence in plants and broad range of biological activities.1 Consequently, efforts to isolate lignans from plant materials2 and for the total synthesis of these compounds have been undertaken.3 From a chemical point of view, lignans show enormous structural diversity, although their molecular backbone consists only of two phenylpropane (C6–C3) units. Bimolecular phenoxy radical coupling reactions are involved in enzymatic and chemical lignan preparation.4 These radicals are also generated by 308 nm laser-induced photoionization of 4-propenyl phenols5 or by photosensitized oxidation.6 In the latter case the work, which aimed to discuss the structures and mechanistic pathways of the obtained products, reported promising results for this reaction when applied to isoeugenol. Starting from these data, in the present work, we have examined the behaviour of isoeugenol and other phenylpropene derivatives (Fig. 1) under various photo-oxidative conditions using a product study approach. The aim was to explore the possibility of finding convenient conditions to prepare lignan-like compounds by environmentally friendly approaches such as photo-induced synthetic methods.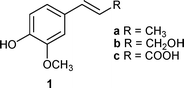 | ||
| Fig. 1 Structures of phenylpropenes. | ||
Experimental
Materials and analysis
Isoeugenol (1a) and ferulic acid (1c) were purchased from Aldrich and used as received. Coniferyl alcohol (1b) was prepared by reduction of ethyl ferulate (which was prepared from acid 1c by esterification with EtOH–HCl) with DIBAL.7 The other chemicals (Fluka) were used without further purification. All solvents (Fluka) were HPLC grade. The methylene blue-sensitized photooxygenations were performed in a 50 ml Pyrex flask, by irradiation with an external 650 W halogen lamp (General Electric). A photoreactor (Helios Italquartz) equipped with a 500 W high-pressure mercury lamp (through a Pyrex glass filter, λ > 300 nm) was used for UV irradiation. Product composition was determined by 1H NMR spectroscopy on the crude reaction mixture, after solvent evaporation and dissolution of the residue in CDCl3 or CD3OD.Methylene blue (MB)-sensitized photooxygenation of isoeugenol (1a)
A solution of 1a (100 mg, 0.61 mmol) and methylene blue (MB, 22 mg, 0.06 mmol) was irradiated at 15 °C with a halogen lamp while dry oxygen was bubbled through the solution. Solvents, concentrations and irradiation times are reported in Table 1. After solvent evaporation, chromatography of each residue on silica gel TLC [eluting with hexane–ethyl acetate 7 : 3] followed by HPLC column RP-18 [H2O–MeOH–MeCN (4 : 3 : 3)] led to compounds 2a, 3, 4, 5, and 6 (Fig. 2) with the yields reported in Table 1. All products were spectroscopically characterized (IR, NMR, MS) and known compounds were identified by comparison of spectral data with those reported.| Conc./M | Solvent/additive/T | Time/h | Isolated yield (%) | |||||
|---|---|---|---|---|---|---|---|---|
| 1a | 2a | 3 | 4 | 5 | 6 | |||
| 0.36 | CH2Cl2/MB/15 °C | 30 | 3 | 30 | 15 | — | 3 | Trace |
| 0.1 | CH2Cl2/MB/15 °C | 12 | 50 | 20 | 10 | — | Trace | Trace |
| 0.36 | CH3CN/MB/15 °C | 30 | 30 | 20 | 10 | — | Trace | Trace |
| 0.1 | CH3CN/MB/15 °C | 12 | 50 | 15 | 15 | — | Trace | — |
| 0.1 | H2O/MB/15 °C | 12 | 50 | 4 | 5 | 20 | Trace | 2 |
| 0.1 | H2O/CH3CN (2 : 1)/MB/15 °C | 12 | 40 | 22 | 8 | 8 | 4 | — |
| 0.1 | CH3CN/(t-BuO)2/r.t. | 14 | 40 | 20 | Trace | — | Trace | — |
| 0.1 | CH3CN/chloranil/r.t. | 8 | 60 | 20 | — | — | — | — |
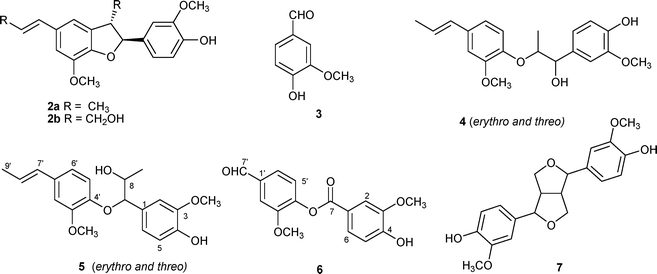 | ||
| Fig. 2 Structures of lignans. | ||
Irradiation of isoeugenol (1a) with di-tert-butyl peroxide
A solution of 1a (100 mg, 0.61 mmol) and 0.23 ml of di-tert-butyl peroxide (two equivalents) in dry CH3CN (0.05 M) was bubbled with a slow stream of argon for 30 min and then irradiated with the UV lamp. After 14 h of irradiation the residue was analyzed by 1H NMR. Chromatography purifications gave 2a (∼20%) and traces of compounds 3 and 5.Irradiation of isoeugenol (1a) with chloranil
A solution of 1a (50 mg, 0.30 mmol) and 24.5 mg of chloranil in dry CH3CN (0.015 M) was bubbled with a slow stream of argon for 30 min and then irradiated with the UV lamp. After 8 h of irradiation the residue was analyzed by 1H NMR. Chromatography purifications gave 2a (∼20%).Methylene blue-sensitized photooxygenation of coniferyl alcohol (1b) and ferulic acid (1c)
Solutions of 1b (100 mg, 0.56 mmol) and 1c (100 mg, 0.52 mmol) in H2O–CH3CN (2 : 1) (0.1 M) were irradiated at 15 °C with the halogen lamp as above described for 1a. During irradiation oxygen was bubbled through the solutions.After 20 h 1H NMR analysis of the residue from 1b indicated the presence of compound 3 as the only product. The 1H NMR spectrum of the residue from 1c showed the presence of starting 1c (80%) and its cis-isomer (20%).
Irradiation of 1b and 1c with di-tert-butyl peroxide
A solution of 1b (50 mg, 0.28 mmol) and 0.11 ml of di-tert-butyl peroxide in dry CH3CN (0.05 M) was bubbled with a slow stream of argon for 30 min and, then, irradiated with the UV lamp. After 14 h of irradiation the residue was analyzed by 1H NMR. The mixture was purified by HPLC [column RP-18, H2O–MeOH–MeCN (5 : 3 : 2)], to give compounds 2b (30%) and 7 (10%).Acid 1c was treated similarly. After 14 h of irradiation, the 1H NMR spectrum of the residue showed the presence of starting 1c (72%) and its cis-isomer (28%).
Results
Isoeugenol (1a) was subjected to methylene blue-sensitized photooxygenation under various conditions (solvent, concentration) (Table 1). Changing the concentration from 0.36 M to lower 0.1 M either in CH3CN or in CH2Cl2 allowed the reaction time to be reduced avoiding secondary oxygenation, mainly of the double bond.8 Moreover, the reaction mixture was less complex and product separation easier. Cheap, safe and non-toxic solvents such as water or water-mixed with CH3CN were also tested. 1H NMR analysis and preparative TLC and HPLC were used to identify and quantify the isolated products. Compound 2a was identified as dehydroisoeugenol by comparison of its spectral data with those reported in the literature.9 Compound 3 was identified as vanillin by comparison with an authentic sample. Compound 4, which was obtained as a 2 : 1 diastereomeric erythro–threo mixture, was identified as guaiacylpropan-1,2-diol-β-O-4′-isoeugenylether by comparison with literature spectroscopic data.10Compound 5 was a 9 : 1 diastereoisomeric mixture as observed in its 1H NMR spectrum. The molecular formula C20H24O5 was deduced by the HREI-MS spectrum which showed the [M]+ ion at m/z 344.1620 (calcd for [C20H24O5]+: 344.1624). Signals (major isomer) at δ 6.29 (1 H, d, J = 15.5 Hz, H-7′), and 6.08 (1 H, dt, J = 15.5, 6.5 Hz, H-8′), in the 1H NMR spectrum, indicated the presence of a trans-substituted double bond. Six aromatic signals in a δ 6.95–6.58 range in the 1H NMR spectrum suggested the presence of two 1,3,4-trisubstituted aromatic rings, as did six methine signals at δ 109.3–120.9 in the 13C NMR (DEPT) spectrum. In the HMBC spectrum, long-range correlations from the H-7′ olefinic proton to the methyl carbon (δ 18.9, C-9′) and the methine carbons C-2′ (δ 109.8), and C-6′ (δ 119.1), were observed. In the 1H NMR spectrum, the signals at δ 4.43 (1 H, d, J = 7.8 Hz, H-7), 4.07 (1 H, m, H-8), and 1.01 (3 H, d, J = 6.0 Hz, H-9), as well as signals at δ 91.0 (C-7), 71.3 (C-8), and 18.0 (C-9) in the 13C NMR (DEPT) spectrum indicated the presence of a propane-1,2-diol chain. In the HMBC spectrum, long-range correlations from the H-7 proton to the methyl (C-9), the methine carbons at δ 71.3, 109.3, 120.9, 131.0, and 145.1 assigned to C-8, C-2, C-6, C-1, and C-4′, respectively were observed, indicating the presence of a 1-(4-hydroxy-3-methoxyphenyl)propane-1,2-diol linked to C-4 of the isoeugenol unit that involve a 4′-O-7 linkage. Compound 5 was previously reported as oxidatively dimerized isoeugenol.11
Compound 6 was isolated from the irradiation mixture in water (2%). It had molecular formula C16H14O6 as the HREI-MS spectrum showed the [M]+ ion at m/z 302.0785 (calcd for [C16H14O6]+: 302.0790). The 1H NMR spectrum showed a singlet at δ 9.98 attributed to an aldehydic group, as confirmed by a signal at δ 192.2 in the 13C NMR spectrum. Six aromatic signals in a δ 7.02–7.84 range, in the 1H NMR spectrum and 1H–1H COSY suggested the presence of two 1,3,4-trisubstituted aromatic rings, as did six methine signals at a δ 111.2–125.8 range in the 13C NMR (DEPT) spectrum. These data, along with HSQC and HMBC spectra, confirmed the structure of compound 6 as depicted.
As shown in Table 1, appreciable amount of lignan 2a was obtained both in organic solvents and in H2O–CH3CN. Compound 4 was found in 20% and 8% amounts in H2O and H2O–CH3CN as solvents. Compound 5 was present in small amounts under all the conditions used.
A rationalization of the results is reported in Scheme 1. In the formation of compounds 2a, 4, 5 the key intermediate should be radical 8a deriving from an electron-transfer reaction between 1a and singlet oxygen followed by hydrogen abstraction.12 Radical couplings and nucleophilic additions are the subsequent events. As reported,62a is formed by 5′-8 coupling followed by an intramolecular cycloaddition. 4′-O-8 Coupling and water addition to the resulting quinone methide appear to lead to compound 4. The low yield can be explained on the basis of previous data,4b which evidenced the scarce competition of water addition with respect to that of other nucleophiles such as methanol or ethanol, even in the presence of excess of water. Oxygenated species such as an OOH radical (or O2)11 may be involved in the formation of compound 5, followed by the nucleophilic addition of 1a.
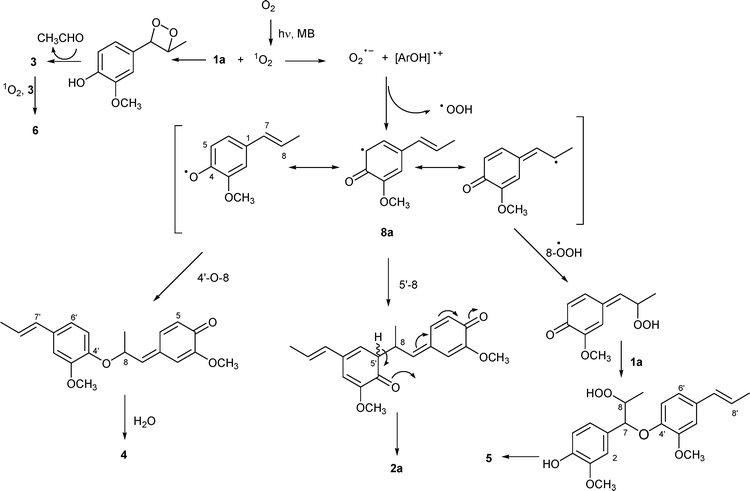 | ||
| Scheme 1 Suggested pathways for compounds 2a–6 under methylene blue (MB)-sensitized photooxygenation. | ||
Vanillin (3) is the cleavage product of the intermediate dioxetane deriving from addition of singlet oxygen to the double bond (Scheme 1).13 The minor product 6, according to Kuo,6 should be formed via abstraction of the phenolic hydrogen from 3 by 1O2, as above for 1a, and addition of the so formed radical to the aldehydic group of 3 followed by hydrogen transfer to radical species lead to the formation of 6.
To minimize oxygen addition we used typical conditions for generating phenoxy radicals by different approaches (Scheme 2).5,14 When isoeugenol (1a) was irradiated at λ > 310 nm in the presence of di-tert-butyl peroxide in dry CH3CN in an inert atmosphere,5 the reaction mixture, analysed by 1H NMR after 14 h, evidenced 60% conversion and the presence of compound 2a (20%) in addition to trace amounts of 3 and 5. Irradiation was also carried out in the presence of chloranil, a well-known electron-transfer sensitizer5 able to abstract hydrogen,14 in dry CH3CN, in an inert atmosphere. After 8 h, the reaction mixture, analysed by 1H NMR, evidenced 40% conversion and the presence of compound 2a only in a 20% amount.
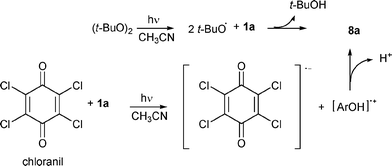 | ||
| Scheme 2 Other photochemical approaches to radical 8a. | ||
The mild conditions (MB-sensitized photooxygenation in H2O–CH3CN and peroxide-promoted photo-oxidation) were also used for coniferyl alcohol (1b) and ferulic acid (1c). The results are depicted in Scheme 3. The photooxygenation of 1b in H2O–CH3CN and methylene blue as a sensitizer led, after 20 h, only to vanillin (3), evidently due to the activated double bond.15 More satisfying was irradiation in the presence of the peroxide. The reaction mixture, analysed after 14 h by 1H NMR, evidenced 90% conversion. HPLC separation gave compounds 2b (30%) and 7 (10%), identified by comparison with literature spectroscopic data as dihydrodiconiferyl alcohol16 and pinoresinol,17 respectively. Radical 8b, formed as above for 8a, partly gives the β-5 dimer 2b and partly dimerizes to the furofuran lignan 7 through initial 8–8′ coupling followed by intramolecular 7-O-9′ and 7′-O-9 cyclizations of alcoholic functions.
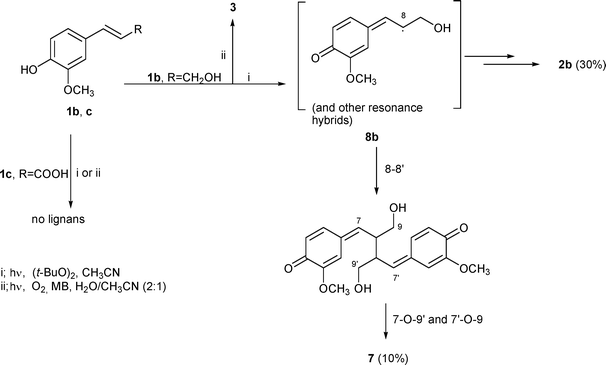 | ||
| Scheme 3 Reactions of coniferyl alcohol (1b) and ferulic acid (1c). | ||
All attempts to oxidise ferulic acid (1c) failed under both conditions. No lignan derivatives were observed even after prolonged irradiation (30 h). Cis-trans-isomerization and, under MB-sensitized photooxygenation, formation of vanillin (trace) were the only events observed.
Conclusions
Formation of cyclic or acyclic lignans under light-promoted oxidation is closely related to the starting propenyl phenols. Although limited, the use of peroxide-mediated irradiation appears to be of interest for lignans 2a, 2b and 7 due to the selective and green conditions used.References
- D. C. Ayres and J. D. Loike, Lignans Chemical, Biological and Clinical Properties, Cambridge University Press, UK, 1990 Search PubMed.
- M. Saleem, H. J. Kim, M. S. Ali and Y. S. Lee, An update on bioactive plant lignans, Nat. Prod. Rep., 2005, 22, 696–716 RSC.
- (a) D. J. Wardrop and J. Fritz, Total Synthesis of (±)-Magnofargesin, Org. Lett., 2006, 8, 3659–3662 CrossRef CAS; (b) G. D. McAllister, M. F. Oswald, R. J. Paxton, S. A. Raw and R. J. K. Taylor, The direct preparation of functionalised cyclopropanes from allylic alcohols or α-hydroxyketones using tandem oxidation processes, Tetrahedron, 2006, 62, 6681–6694 CrossRef CAS; (c) J.-C. Kim, K.-H. Kim, J.-C. Jung and O.-S. Park, An efficient asymmetric synthesis of furofuran lignans: (+)-sesamin and (–)-sesamin, Tetrahedron: Asymmetry, 2006, 17, 3–6 CrossRef CAS.
- (a) J. B. Iqbal, B. Bhatia and N. K. Nayyar, Transition metal-promoted free-radical reactions in organic synthesis: the formation of carbon–carbon bonds, Chem. Rev., 1994, 94, 519–564 CrossRef CAS; (b) F. Chioccara, S. Poli, B. Rindone, T. Pilati, G. Brunow, P. Pietikainen and H. Setala, Regio- and Diastereo-selective Synthesis of Dimeric Lignans using Oxidative Coupling, Acta Chem. Scand., 1993, 47, 610–616 CrossRef CAS; (c) K. Freudenberg, Lignin. Its constitution and formation from p-hydroxycinnamyl alcohols, Science, 1965, 148, 595–600 CrossRef CAS.
- Y. Rodriguez-Evora and N. P. Schepp, Reactivity of 4-vinylphenol radical cations in solution: implications for the biosynthesis of lignans, Org. Biomol. Chem., 2005, 3, 4444–4449 RSC.
- Y.-H. Kuo, L.-H. Chen and L.-M. Wang, Photosensitized oxidation of isoeugenol in protic and aprotic solvents, Chem. Pharm. Bull., 1991, 39, 2196–2200 CAS.
- S. Quideau and J. Ralph, Facile large-scale synthesis of coniferyl, sinapyl, and p-coumaryl alcohol, J. Agric. Food Chem., 1992, 40, 1108–1110 CrossRef CAS.
- Low power lamps (3 × 20 W) and hence long irradiation times (5 d) are the conditions used in Ref. 6.
- I. R. Nascimento and L. M. X. Lopes, 2,3-Dihydrobenzofuran neolignans from Aristolochia pubescens, Phytochemistry, 1999, 52, 345–350 CrossRef CAS.
- S. Hada, M. Hattori, Y. Tezuka, T. Kikuchi and T. Namba, New neolignans and lignans from the aril of Myristica fragrans, Phytochemistry, 1988, 27, 563–568 CrossRef CAS.
- Y. Oka, A. Nakamura, H. Watanabe and K. Touhara, An odorant derivative as an antagonist for an olfactory receptor, Chem. Sens., 2004, 29, 815–822 Search PubMed.
- (a) F. Cermola, M. DellaGreca, M. R. Iesce, S. Montella, A. Pollio and F. Temussi, A mild photochemical approach to the degradation of phenols from olive oil mill wastewater, Chemosphere, 2004, 55, 1035–1041 CrossRef CAS; (b) T. Matsuura, N. Yoshimura, A. Nishinaga and I. Saito, Photoinduced reaction. LVI. Participation of singlet oxygen in the hydrogen abstraction from a phenol in the photosensitized oxygenation, Tetrahedron, 1972, 28, 4933–4938 CrossRef CAS.
- Dioxetanes are labile intermediates and undergo easily O–O and C–C bonds cleavage giving carbonyl compounds, M. R. Iesce, in Synthetic Organic Photochemistry, ed. A. G. Griesbeck and J. Mattay, Marcel Dekker, New York, USA, 2005, vol. 12, pp. 299–363. In this case dioxetane leads to 3 and volatile non-detectable CH3CHO Search PubMed.
- H. J. P. de Lijser and N. A. Rangel, Photochemical acetalization of carbonyl compounds in protic media using an in situ generated photocatalyst, J. Org. Chem., 2004, 69, 8315–8322 CrossRef CAS.
- M. Matsumoto, H. Kobayashi, J. Matsubara, N. Watanabe, S. Yamashita, D. Oguma, Y. Kitano and H. Ikawa, Effect of Allylic Oxygen on the Reaction Pathways of Singlet Oxygenation: Selective Formation of 1,2-Dioxetanes from 1-Alkoxymethyl-2-aryl-1-tert-butyl-2-methoxyethylenes, Tetrahedron Lett., 1996, 37, 397–400 CrossRef CAS.
- M. S. M. Yuen, F. Xue, T. C. W. Mak and H. N. C. Wong, On the absolute structure of optically active neo-lignans containing a dihydrobenzo[b]furan skeleton, Tetrahedron, 1998, 54, 12429–12444 CrossRef CAS.
- M. Miyatzawa, K. Hiroyuki and H. Kameoka, Phenolic lignans from flower buds of Magnolia fargesii, Phytochemistry, 1992, 31, 3666–3668 CrossRef.
| This journal is © The Royal Society of Chemistry and Owner Societies 2008 |
