Experimental data on the stereoselectivity of base-catalyzed 1,2-elimination reactions that produce conjugated carbonyl compounds are scarce in spite of the importance of these reactions in organic and biochemistry. As part of a comprehensive study in this area, we have synthesized stereospecifically-deuterated β-tosyloxybutanoate esters and thioesters and studied the stereoselectivity of their elimination reactions under non-ion pairing conditions. With the availability of both the (2R*,3R*) and (2R*,3S*) diastereomers the innate stereoselectivity could be determined unambiguously. 1H and 2H NMR data show that these substrates produce 5–6% syn elimination, the usual amount for acyclic substrates undergoing E2 reactions. Contrary to earlier suggestions, activation by a carbonyl group has virtually no influence upon the stereoselectivity. Elimination of the (2R*,3R*) diastereomer of the β-tosyloxyester and thioester produces 21–25% of the (Z)-alkene, much more than observed with a poorer β-nucleofuge. A relatively large amount of (Z)-alkene product seems to be a good marker for an E2 pathway, in which the transition state is E1cB-like, rather than an E1cBirrev mechanism. Syn KIE values were higher than those for anti elimination for the esters as well as the thioesters. Experimental challenges to the synthesis of stereospecifically-deuterated β-tosyloxyesters are discussed.
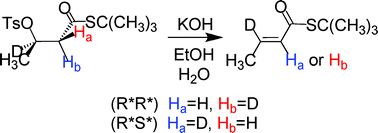
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
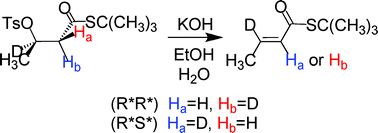

 Please wait while we load your content...
Please wait while we load your content...