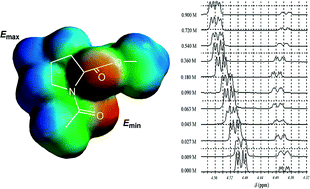
1H NMR titration experiments have been used to establish that minimal proline-based models show enhanced binding selectivity towards phenol in CDCl3, relative to other similarly protected amino acid residues. Cooperative binding effects appear to play a role, with sarcosine models affording binding constants to phenol intermediate to those obtained from proline models and other amino acid models. The mechanism for binding, based on DFT calculations and the application of Hunter's molecular recognition toolbox model, cannot be solely attributed to hydrogen bond strength, and appears to be mediated through C–H-π bonds and the rotational freedom of the amide substrate.