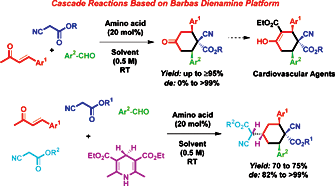
The amino acid proline catalyzed the three- and five-component cascade olefination–Diels–Alder–epimerization and olefination–Diels–Alder–epimerization–olefination–hydrogenation reactions of readily available precursors enones 1a–i, arylaldehydes 2a–k, alkyl cyanoacetates 3a–e and Hantzsch ester 9 to furnish highly substituted prochiral 1-cyano-4-oxo-2,6-diaryl-cyclohexanecarboxylic acid alkyl esters 6 and 1-cyano-4-(cyano-alkoxycarbonyl-methyl)-2,6-diaryl-cyclohexanecarboxylic acid alkyl esters 10 in a highly diastereoselective fashion with excellent yields. Prochiral cis-isomers 6 are excellent starting materials for the synthesis of cardiovascular agents and hypnotic active products.