A comparison of orbital interactions in the additions of phosphonyl and acyl radicals to double bonds†
Abstract
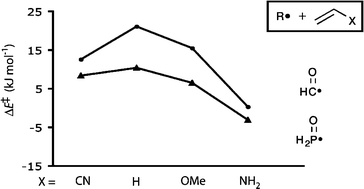
Calculation of the barriers for addition of the H2P(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)˙ and HC(
O)˙ and HC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)˙ radicals to
O)˙ radicals to ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)˙ radical this behaviour occurs because a secondary orbital interaction of the type π*C
O)˙ radical this behaviour occurs because a secondary orbital interaction of the type π*C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O←HOMO acts in conjunction with the primary SOMO←HOMO interaction to balance the SOMO→LUMO interaction. For the H2P(
O←HOMO acts in conjunction with the primary SOMO←HOMO interaction to balance the SOMO→LUMO interaction. For the H2P(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)˙ radical, on the other hand, the much higher-lying LUMO (the σ*P–O orbital) allows for only minimal secondary interaction, and this radical's ambiphilic behaviour is therefore reflective of a balance between SOMO→LUMO and SOMO←HOMO interactions.
O)˙ radical, on the other hand, the much higher-lying LUMO (the σ*P–O orbital) allows for only minimal secondary interaction, and this radical's ambiphilic behaviour is therefore reflective of a balance between SOMO→LUMO and SOMO←HOMO interactions.

 Please wait while we load your content...
Please wait while we load your content...