Themed issue: Polymorphism and crystal forms
The quest for, and the identification and characterization of, different crystal forms (polymorphs, solvates) of the same molecule, or of aggregates of the same molecule with other molecules or ions (co-crystals and salts), is one of the most active and challenging research areas of modern solid-state chemistry (Fig. 1).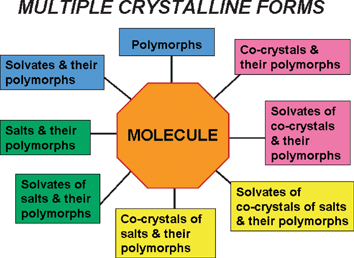 | ||
| Fig. 1 Schematic representation of the multiple crystalline forms that can be generated from the same molecule. | ||
Crystal polymorphism,1 resulting from different packing arrangements of the same molecular or supramolecular entity in the crystal structure, represents a challenge to crystal-makers.2 As a matter of fact, the paradigm of molecular crystal engineering3 is based on the very idea of rational design, and the construction of crystalline solids with pre-defined architectures and physical properties, starting from the choice of molecular components.
Why are polymorphism and multiple crystal forms important? Different crystal forms of a substance can possess very different properties and behave as different materials. Polymorphs can manifest different properties, such as physical, thermodynamic, spectroscopic, kinetic, surface, mechanical and chemical properties. This awareness has important implications in all fields of chemistry associated with the production and commercialization of molecules in the form of crystalline materials (drugs, agrochemicals, food additives, pigments, explosives, etc.).1
The last decade has witnessed many developments in the design and characterization of new crystal forms. The reasons for this are to be found in the increased awareness of the possibility of multiple crystal forms of a substance, the utility that may be derived from preparing a crystal form with enhanced properties, and the potential intellectual property implications of new crystal forms, combined with the development of new technology4 and attempts to design and control crystal structure.5 New crystallization techniques have also been developed to obtain new crystal forms,6 which can then be characterized in the solid-state. Several solid-state techniques are used, and others are being developed, to help in this difficult task; among them, we find (but the list is far from exhaustive) microscopy and hot stage microscopy (HSM), differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), infrared (IR) and Raman spectroscopy, single-crystal/powder X-ray diffraction (SCXRD, XRPD), and solid-state nuclear magnetic resonance spectroscopy (SSNMR). There is also a strong interest in being able to compute and predict the relative stability of different crystal forms, and the potential existence of polymorphism.7
However, our ability to predict or control the occurrence of polymorphism is still embryonic. In many cases, the crystallization of a new crystal form, or of an amorphous phase of a given substance, turns out to be the result of serendipity8 rather than a process under complete human control.
In addition to this, crystallization remains a challenging issue. We are still not able to predict the shape, size or thermodynamic stability of a new solid chemical, whether its crystals will be thermodynamically stable or metastable, whether they will undergo phase changes with temperature or pressure, include solvent molecules upon crystallization, or whether such solvents could be removed or replaced without destroying the crystal edifice.
The widely quoted statement of McCrone, “It is at least this author’s opinion that every compound has different polymorphic forms and that, in general, the number of forms known for a given compound is proportional to the time and energy spent in research on that compound”,9 seems to suggest that polymorphism is the rule, not the exception. On the contrary, in spite of the growing awareness and economic importance of polymorphism, most documented cases have been discovered by serendipity rather than through systematic searches. Polymorphism is always possible, but the conditions necessary to obtain polymorphic forms of a given substance are not yet obvious. The a priori prediction of crystal polymorphism and crystal forms is still a great challenge, as not even the most thorough polymorph screening can guarantee, above all doubts, that the most thermodynamically stable crystals of a given species have ultimately been obtained.
The scope of this issue is that of providing to the reader a glimpse of the phenomenon of multiple crystals forms in general and polymorphism in particular, a fascinating and rapidly developing area of solid-state chemistry, and a field that will provide many scientific—and economic—challenges in future years.
Fabrizia Grepioni
(Università di Bologna)
References
- J. Bernstein, Polymorphism in Molecular Crystals, Oxford University Press, Oxford, 2002 Search PubMed.
- Making Crystals by Design: Methods, Techniques and Applications, ed. D. Braga and F. Grepioni, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2007 Search PubMed.
- (a) G. R. Desiraju, Crystal Engineering: The Design of Organic Solids, Elsevier, Amsterdam, 1989 Search PubMed; (b) Crystal Engineering: From Molecules and Crystals to Materials, ed. D. Braga, F. Grepioni and A. G. Orpen, Kluwer Academic Publishers, Dordrecht, 1999 Search PubMed; (c) D. Braga, Chem. Commun., 2003, 2751 RSC.
- S. L. Morissette, S. Soukasenem, D. Levinson, M. J. Cima and Ö. Almarsson, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 2180 CrossRef CAS.
- (a) Crystal Design: Structure and Function, ed. G. R. Desiraju, Wiley, Chichester, 2003 Search PubMed; (b) D. Braga and F. Grepioni, Angew. Chem., Int. Ed., 2004, 43, 4002 CrossRef CAS.
- J. Bernstein, Chem. Commun., 2005, 5007 RSC.
- S. L. Price, Phys. Chem. Chem. Phys., 2008, 10, 1996 RSC.
- R. K. Merton and E. Barber, The Travels and Adventures of Serendipity, Princeton University Press, Princeton, 2004 Search PubMed.
- W. C. McCrone, in Polymorphism in Physics and Chemistry of the Organic Solid State, ed. D. Fox, M. M. Labes and A. Weissenberg, Interscience, New York, 1965, vol. II, pp. 726 Search PubMed.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2008 |
