Demonstration of a bio-microactuator powered by vascular smooth muscle cells coupled to polymer micropillars
Yo
Tanaka
ab,
Kae
Sato
c,
Tatsuya
Shimizu
bd,
Masayuki
Yamato
bd,
Teruo
Okano
bd,
Ichiro
Manabe
e,
Ryozo
Nagai
e and
Takehiko
Kitamori
*abc
aDepartment of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 113-8656, Japan. E-mail: kitamori@icl.t.u-tokyo.ac.jp; Fax: +81 3-5841-6039; Tel: +81 3-5841-7231
bCore Research for Evolutional Science and Technology (CREST), Japan Science and Technology Agency, Kawaguchi, Saitama, 332-0012, Japan
cCenter for NanoBio Integration, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 113-8656, Japan
dInstitute of Advanced Biomedical Engineering and Science, Tokyo Women's Medical University, 8-1 Kawada-cho, Shinjuku-ku, Tokyo, 162-8666, Japan
eDepartment of Cardiovascular Medicine and Molecular Pathology, School of Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 113-8656, Japan
First published on 22nd November 2007
Abstract
We have demonstrated the working principle of a bio-microactuator using smooth muscle cells (SMCs) by driving micropillars coupled to cultured SMCs and controlled pillar displacements by chemical stimuli; the generated driving force was estimated to be over 1.1 µN.
Integrated chemical systems, also known as micro total analysis systems (µ-TAS) or labs-on-a-chip, are currently a major interest due to their desirable characteristics, including reductions in reagent consumption, space requirements and analysis times.1–3 To drive fluids in micro chemical devices, microelectromechanical system (MEMS) fluidic devices are usually used. On the other hand, a circulatory system in a living body can operate fluids effectively in a microspace without electricity. To realize micro chemical systems powered without a need for external power sources, we have proposed to exploit this sophisticated biological function to operate fluids with only chemical energy input, and developed bio-microactuators utilizing spontaneously and synchronously beating cardiomyocytes.
To date, we have demonstrated the concept of a cardiomyocyte bio-microactuator by driving microstructures,4 and also developed a pump using cardiomyocytes5 and a micro-spherical heart pump6 as practical microfluidic devices. Moreover, other groups have also fabricated a cardiomyocyte pump7 and robots.8–10
However, the actuation of cardiomyocytes is difficult to control because they beat spontaneously, and therefore, cardiomyocyte bio-microactuators cannot be applied to flow control devices such as valves or fluid pressure regulators for which displacement and force must be controlled by outside operations like normal electrical actuators. Here, we focus on a vascular blood flow control function and utilize vascular smooth muscle cells (SMCs) to realize a complete cell-based microfluidic device including flow control functions. SMCs surrounding blood vessels contract or relax to maintain the optimal blood flow pressure by responding to signal molecules secreted by endothelial cells (ECs) that line the inner vessels and that sense changes in shear stress levels present in their environment. By exploiting SMCs for which displacement can be controlled by chemical stimuli from outside and that can keep contracting or relaxing for a relatively long time (order of minutes), flow control devices requiring continuous force to remain stopped or to regulate fluid flow should be realized. To develop such an SMC device, it is indispensable to demonstrate bio-microactuation using SMCs and their displacement control against chemical stimuli. Although SMC local forces have been measured using microdevices such as a micropillar array11,12 or beads embedded gel,13 they were static forces and the SMCs did not work as actuators per se.
The objective of this communication is to demonstrate the working principle of an SMC-based bio-microactuator by driving fabricated microstructures using attached SMCs. First, pillar-shaped structures are micro-fabricated and actuated by an attached SMC contraction. Second, to demonstrate displacement control of the bio-microactuator, actuation of a pillar by both SMC contraction and relaxation is demonstrated. Finally, forces generated by attached SMCs for observed driven micropillars are estimated.
A micropillar is actuated by chemical stimuli as described in Fig. 1. The intrinsic self-expansion capabilities of cells permit direct growth onto the microstructures in situ without any associated damage;4 this differs from conventional measurement methods of single cellular contractile forces by direct attachment of cells onto force transducers using a micromanipulator.14,15 The contractile force is estimated from the displacement of flexible polymer pillars made of poly(dimethylsiloxane) (PDMS). For small deflections, a pillar can behave as an ideal, simple Hookean spring such that the pillar deflection is directly proportional to the force generated by attached cells. For a rectangular pillar, this behavior is described by eqn (1):4
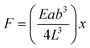 | (1) |
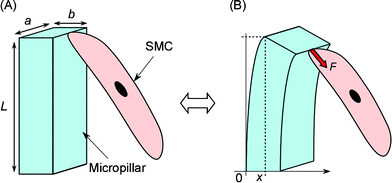 | ||
| Fig. 1 Actuating principle of a micropillar coupled to and powered by a cultured SMC. The micropillar is pulled and returned by the motion of the attached SMC against chemical stimuli. (A) The SMC is relaxing. (B) The SMC is contracting. a, b, L are the width, thickness, height of the pillar, respectively. F is the bending force, x is the pillar displacement. | ||
Fabricating procedures for rectangular PDMS micropillars were described previously.4 Briefly, a silicon wafer was patterned with a lattice, leaving an array of micropillars at the center of the wafer. PDMS prepolymer was poured over the wafer, cured and peeled off to get a mold. Next, prepolymer was poured over the mold, cured, peeled off to get a PDMS sheet embedded with an array of micropillars. The sheet was then cut into small square pieces and one of them was placed into a cell culture dish (35 mm diameter). The PDMS piece was sterilized using UV light for 15 min and immersed for 2 h in 10 µg mL–1laminin (bovine serum-derived, Sigma) solution in phosphate buffered saline (PBS) at 37 °C to promote pillar attachment by SMCs. The size of each micropillar was 10 µm × 15 µm (cross section) × 25 µm (height). These pillars were utilized to compare the results with previous ones obtained by using the same aspect of pillars actuated by cardiomyocytes.4
Next, SMCs were prepared. Rat aortic SMCs (RASMCs) were harvested from male Sprague–Dawley rats as described previously,16 and cultured in the SMC culture medium consisting of DMEM + F12 (1 : 1) (Sigma) supplemented with 10% FBS (HyClone) and 1% penicillin–streptomycin (Sigma) solution. After RASMCs became confluent, they were washed with 6 mL of PBS and detached from the dish by immersing them in 4 mL of trypsin EDTA (Invitrogen) for 3 min. Detached RASMCs were rinsed with 6 mL of PBS and added to 5 mL of the medium in a Falcon tube, and the cell suspension was centrifuged at 1100 rpm for 5 min. Finally, the supernatant was aspirated and RASMCs were re-suspended in the medium at a density of 5 × 105cells mL–1 and some portion (3 mL per dish) was poured into the dish containing the treated PDMS sheet. Incubation and culture were performed at 37 °C in a humidified atmosphere with 5% CO2.
The cultured RASMCs reached confluence on the PDMS sheet within 4 days and some were observed to attach onto the micropillars heterogeneously (Fig. 2A and B). After that, the medium was replaced to serum-free medium (SFM) consisting of DMEM + F12 (1 : 1) supplemented with 1% penicillin–streptomycin solution, 35.2 mg L–1ascorbic acid (Sigma), 5.0 mg L–1 transferrin (Sigma), 2.85 mg L–1insulin (Sigma) and 6.25 µg L–1selenium (Sigma) to prevent the presence of SMC contraction factors contained in serum. Culturing was continued for 3 more days (total 7 days). The cultured SMCs and micropillars were observed using a phase contrast microscope (ELIPSE TE300, Nikon) at 37 °C with an objective lens (60×, 0.70-NA, 0.4 µm-resolution). The microscope was focused on the pillar tops and the image was recorded by an interfaced video cassette recorder (WV-DR9, Sony) through a CCD camera (HV-D28S, Nikon) during the observation.
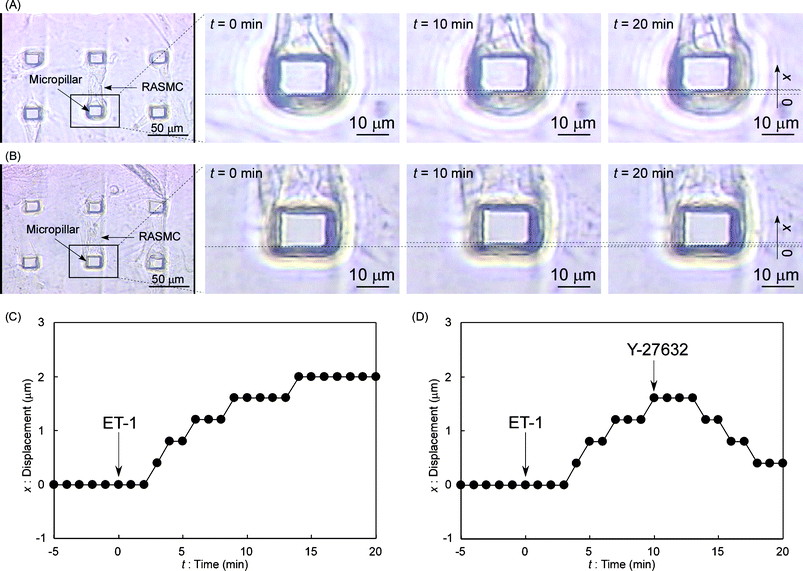 | ||
| Fig. 2 Observation of the pillar actuation by RASMCs. (A) Video images of the driving micropillar by RASMC contraction. An attached RASMC was relaxed at t = 0 s (just after ET-1 stimulation). The pillar was gradually driven in the upwards direction of the photos during 20 min (x = 2.0 ± 0.2 µm at t = 20 min). (B) Video images of the driving micropillar by both RASMC contraction and relaxation. The attached RASMC was relaxed at t = 0 s (just after ET-1 stimulation). The pillar was gradually driven in the upwards direction of the photos during 10 min (x = 1.6 ± 0.2 µm at t = 10 min). After Y-27632 stimulation at t = 10 min, the pillar was gradually returned to nearly its original position during the next 10 min (x = 0.4 ± 0.2 µm at t = 20 min). (C) Displacement time-course for the driving pillar in part A during a 25 min period. (D) Displacement time-course for the driving pillar in part B during a 25 min period. | ||
First, a micropillar was actuated by an attached SMC contraction using an EC-derived SMC contractile peptide, endothelin-1 (ET-1).17 A pillar on which a cell was attached to one side was chosen for observation. Five minutes after starting recording, ET-1 (Sigma) SFM solution was added using a micropipette to get a concentration of 1 µM in the dish to stimulate and make RASMCs contract. Fig. 2A shows video frames with the selected pillar and the corresponding pillar displacement kinetics is plotted in Fig. 2C. Although the movement of the pillar was slow, the video frames surely showed the pillar was pulled by attached RASMCs during the 20 min period. It took a few minutes until the contraction began probably because of the spreading time of ET-1. Pillar top displacement (x) was measured directly from sequential video frames of the microscopic video image around the top of the driving micropillar every 1 min. Displacement just after ET-1 stimulation (t = 0 s) was defined as x = 0 µm. Measured points of displacement obtained from images contain ±0.2 µm errors. At t = 20 min, x = 2.0 ± 0.2 µm. To apply SMCs for actuators, it is indispensable that contracted SMCs can be relaxed also by a chemical stimulation. In the second experiment, we used a synthesized peptide for SMC relaxation, Rho-associated kinase inhibitor, Y-27632 (Sigma)18 to demonstrate relaxation. At t = 0 min, ET-1 SFM solution was added to get a concentration of 1 µM in the dish. At t = 10 min, Y-27632 SFM solution was added using a micropipette to get a concentration of 10 µM in the dish, because the displacement of a pillar reached a plateau around t = 10 min in the previous experiment. Fig. 2B shows video frames with the selected pillar and the corresponding pillar displacement kinetics is plotted in Fig. 2D. At t = 10 min, x = 1.6 ± 0.2 µm. At t = 20 min, x had decreased to 0.4 ± 0.2 µm. The pillar did not return to the original position (x = 0 µm), because Y-27632 at this concentration (10 µM) could not inhibit the ET-1 effect completely. However, the pillar would return to the original position using a higher Y-27632 concentration.18 Therefore, micropillar displacements could be controlled by the strength of chemical stimuli.
From these results, for the first time we found that RASMCs could both drive and return microstructures with only chemical stimuli. Since these displacement control operations are fundamental and indispensable for most actuators, the possibility to exploit RASMCs as actuators was demonstrated. In this experiment, actual attachment rate and whether only one cell attached to a pillar were not known. Probably, it is difficult to control these factors in this system. To create actual microfluidic devices using SMCs, the design must not be dependent on these factors. Also, the displacement results of the observed pillars were not repeatable. To demonstrate possibility of actuation of microstructures by SMCs, we used strong and effective chemicals for contraction and relaxation (ET-1 and Y-27632). To demonstrate repeatability, chemicals and their concentration must be reconsidered.
The force of RASMCs stimulated by ET-1 in the former experiment was estimated from the pillar displacement and eqn (1). Since it was not known how cells actually attached to pillars, the contractile force was estimated as minimum (when cells attached to the top of pillars as in Fig. 1). In this estimation, the following values were used: E = 2.5 MPa,11a = 15 µm, b = 10 µm, L = 25 µm and x = 2.0 ± 0.2 µm (at t = 20 min). F was calculated as 1.2 ± 0.1 µN if RASMC forces were applied to the top of the pillar. However, RASMCs might also attach to the pillar sides. Or, the direction of the force might not be vertical to the pillar. In these cases, RASMCs must generate stronger forces than 1.1 µN. Besides, although the shape of the real micropillars was not rectangular but rather pyramidal,4 the force for the simplified rectangular pillar was estimated as smaller than that for the real pyramidal one, and therefore the force generated by RASMCs was over 1.1 µN.
This force was compared with a previously reported contractile force of vascular SMCs in the field of physiology. A single SMC force contracted by Ca2+ was measured to be 5.7 ± 3.1 µN,14 which is on the same order with the present finding (1.1 µN); but we could not decide whether pillars were driven by one or a few cells from obtained images because of the complex shapes of the cultured RASMCs in the present system. We also estimated the force for cardiomyocytes by the same method previously (3.5 µN)4 and it was on the same order as the present finding. Therefore, just as our cardiomyocyte pump was designed and fabricated based on that result,5,6 so too should SMC-based microfluidic devices also be created. To do that, structures and materials must be well considered to transmit the cellular weak force to fluids. Our developed cell sheets19 should also be helpful to obtain larger forces.
In this communication, the working principle of an SMC-based bio-microactuator was demonstrated by driving micropillars using attached SMCs, and pillar displacements could be controlled by chemical stimuli. The maximum pillar displacement was 2.0 ± 0.2 µm, and the corresponding generated force was estimated at over 1.1 µN. However, there are no control experiments of the pillar actuation using non-stimulated SMCs. Perhaps, a micropillar could be pulled a little by an attached SMC in 25 min even without stimulation. Control experiments will be included in a future paper. In blood vessels, ECs work as fluid sensors and reactors, and SMCs work as fluid valves or regulators. Thus, by combining the SMC-based actuator with the ECs-incorporated microdevice we developed previously,20 self-feedback flow control functions can be incorporated into cardiomyocyte pumps whose flow rate and pressure are often fluctuated day by day.6 For this purpose, slow actuation speed of SMCs is allowed. Since SMCs stay active for several days,21 that will be possible. If such complete cell-based microfluidic devices are realized, they will be unique not only because they can work without electricity but also because they can self-regulate flow without any external stimuli.
Acknowledgements
Y. Tanaka was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS).Notes and references
- G. M. Whitesides, Nature, 2006, 442, 368–373 CrossRef CAS.
- P. S. Dittrich, K. Tachikawa and A. Manz, Anal. Chem., 2006, 78, 3887–3908 CrossRef CAS.
- T. Kitamori, M. Tokeshi, A. Hibara and K. Sato, Anal. Chem., 2004, 76, 52A–60A CAS.
- Y. Tanaka, K. Morishima, T. Shimizu, A. Kikuchi, M. Yamato, T. Okano and T. Kitamori, Lab Chip, 2006, 6, 230–235 RSC.
- Y. Tanaka, K. Morishima, T. Shimizu, A. Kikuchi, M. Yamato, T. Okano and T. Kitamori, Lab Chip, 2006, 6, 362–368 RSC.
- Y. Tanaka, K. Sato, T. Shimizu, M. Yamato, T. Okano and T. Kitamori, Lab Chip, 2007, 7, 207–212 RSC.
- J. Park, I. C. Kim, J. Baek, M. Cha, J. Kim, S. Park, J. Lee and B. Kim, Lab Chip, 2007, 7, 1367–1370 RSC.
- J. Kim, J. Park, S. Yang, J. Baek, B. Kim, S. H. Lee, E. S. Yoon, K. Chun and S. Park, Lab Chip, 2007, 7, 1504–1508 RSC.
- J. Xi, J. J. Schmidt and C. D. Montemagno, Nat. Mater., 2005, 4, 180–184 CrossRef CAS.
- A. W. Feinberg, A. Feigel, S. S. Shevkoplyas, S. Sheehy, G. M. Whitesides and K. K. Parker, Science, 2007, 317, 1366–1370 CrossRef CAS.
- J. L. Tan, J. Tien, D. M. Pirone, D. S. Gray, K. Bhadriraju and C. S. Chen, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 1484–1489 CrossRef CAS.
- K. A. Addae-Mensah, N. J. Kassebaum, M. J. Bowers, II, R. S. Reiserer, S. J. Rosenthal, P. E. Moore and J. P. Wikswo, Sens. Actuators, A, 2007, 136, 385–397 CrossRef.
- I. M. Tolic-Norrelykke and N. Wang, J. Biomech., 2005, 38, 1405–1412 CrossRef.
- P. G. Smith, C. Roy, S. Fisher, Q.-Q. Huang and F. Brozovich, J. Appl. Physiol., 2000, 89, 2092–2098 CAS.
- G.-H. Shue and F. V. Brozovich, Biophys. J., 1999, 76, 2361–2369 CrossRef CAS.
- I. Manabe and S. K. Owens, J. Biol. Chem., 2001, 276, 39076–39087 CrossRef CAS.
- M. Yanagisawa, H. Kurihara, S. Kimura, Y. Tomobe, M. Kobayashi, Y. Mitsui, Y. Yazaki, K. Goto and T. Masaki, Nature, 1988, 332, 411–415 CrossRef CAS.
- M. Uehata, T. Ishizaki, H. Satoh, T. Ono, T. Kawahara, T. Morishita, H. Tamakawa, K. Yamagami, J. Inui, M. Maekawa and S. Narumiya, Nature, 1997, 389, 990–994 CrossRef CAS.
- T. Shimizu, M. Yamato, A. Kikuchi and T. Okano, Biomaterials, 2003, 24, 2309–2316 CrossRef CAS.
- Y. Tanaka, Y. Kikukawa, K. Sato, Y. Sugii and T. Kitamori, Anal. Sci., 2007, 23, 261–266 CrossRef.
- K. Oishi, Y. Itoh, Y. Isshiki, C. Kai, Y. Takeda, K. Yamamura, H. Takano-Ohmuro and M. K. Uchida, Am. J. Physiol., 2000, 279, C1432–C1442 CAS.
| This journal is © The Royal Society of Chemistry 2008 |
