Controlled synthesis of rare earth nanostructures
Zheng-Guang
Yan
and
Chun-Hua
Yan
*
Beijing National Laboratory for Molecular Sciences, State Key Laboratory of Rare Earth Materials Chemistry and Applications, PKU-HKU Joint Laboratory in Rare Earth Materials and Bioinorganic Chemistry, Peking University, Beijing. E-mail: yan@pku.edu.cn; Fax: +86-10-6275-4179; Tel: +86-10-6275-4179
First published on 29th September 2008
Abstract
Rare earth compounds form a large family of functional materials with diverse applications in electric, magnetic, optical, and catalytic fields, originating from their unique 4f electrons. Rare earth nanocrystals stir significant interest with their special properties, such as reliable optical applications and enhanced catalytic performances, allowing them to serve as building blocks to construct functional assemblies. In this feature article, we highlight recent works on controlled synthesis of rare earth nanostructures through solution-based routes such as hydrothermal/solvothermal methods and precipitation in high-boiling solvents. Various rare earth nanostructures are obtained both by rationally utilizing intrinsic crystal structures and by fine tuning of experimental conditions, such as temperature, acidity, and capping agents. The controlled phase, crystal growth process, and post-treatment are briefly discussed with typical examples. Rare earth nanocrystals exhibit a variety of shapes and thus are of great benefit for the construction of nanocrystal superlattices. Through the simulation of coarse-grained molecular dynamics, the nanocrystals superstructures are also modelled.
 Zheng-Guang Yan | Zheng-Guang Yan received his PhD degree in 2004 from PKU. Since then, he has worked as a postdoctoral fellow with Prof. Chun-Hua Yan. His main research interests cover the synthesis and simulation of rare earth and other inorganic nanomaterials. |
 Chun-Hua Yan | Chun-Hua Yan is Cheung Kong Professor of Chemistry and the Director of the State Key Laboratory of Rare Earth Materials Chemistry at Peking University (PKU, China). He serves as the Vice President of Chinese Rare Earth Society, Managing Editor-in-Chief for J. Rare Earths and Editor for Mater. Res. Bull. His main research fields are rare earth functional nanomaterials and molecular-based materials. |
1. Introduction
In the past decade, controlled synthesis of functional nanocrystals with pure phase, desirable composition, uniform morphology, and tunable surfaces has become one of the essential topics in materials science. Important nano-materials including carbon nanotubes, semiconductors, noble metals and metal oxides have been extensively studied.1–6 These nanocrystals are usually applied as isolated particles or components of advanced assemblies.The successful applications of rare earth bulk materials as phosphors, magnets, catalysts, superconductors, electrolytes, and electrode materials in solid-state fuel cells, hard alloys, etc.,7–12 have inspired great research interest in their nanoscale counterparts. Unlike the quantum dots, the localized wave function of the rare earth ions makes the typical line spectra based on intra-4f transitions independent of the size or shape of the nanocrystals. Reducing the size to a few nanometres, rare earth nanocrystals may exhibit electron–photon interaction related spectral behaviour as has been observed in rare earth oxides. Specifically, the luminescence spectra related to 4f–5d transitions which involve broad spectral linewidth as occurred for low valence rare earth ions are crystal field related and can be tuned by the size and crystal structure.13 The luminescent properties of rare earth nanocrystals endow better shape tailoring ability to improve nanodevice fabrication. In addition, nanostructured rare earth catalysts exhibit enhanced performance due to the increased surface areas.
In view of the rapidly growing numbers of reports in this domain, it is necessary to clarify the development and prospects in controlled synthesis of rare earth nanocrystals. A comprehensive feature article presented by Boilot and co-workers focused on aqueous synthesis of rare earth colloidal nano-phosphors and their luminescent characteristics.14 In the present feature article, we highlight recent works on rare earth nanostructures, including the synthetic strategies, morphology manipulation, and assembly behaviours of the nano-building blocks.
Conventionally, many rare earth-based phosphors, complex oxides, and sulfides are synthesized through solid state reactions at high temperatures. Nevertheless, solution-based routes featuring low temperatures, simple apparatus, easy control, and versatile post-treatments are always the most important strategies. Among them, hydro- and solvo-thermal methods, and precipitation from high-boiling solvents are widely applied. The experimental conditions, such as temperature, acidity, surfactant, concentration, and reaction time, can be fine-tuned to tailor the rare earth nanostructures.
Despite the factors described above, the intrinsic properties of rare earth compounds are predominant for both phase and morphology. Most simple rare earth inorganic compounds are typically ionic ones, and therefore, relatively rigorous conditions are often required to achieve high crystallinity. There are often polymorphs for rare earth compounds, which show interesting systematic transitions depending on the cationic radii.
As for solution-based fabrication, insoluble oxysalts, fluorides, and hydroxides can be readily precipitated from aqueous solution and the corresponding nanocrystals can be shaped from the colloidal precipitates. In contrast, oxides and sulfides, except ceria, often require quite rigorous conditions and special strategies. The low-valence rare earth compounds containing Eu(II), Yb(II), or Ce(III) are often prepared with protection against oxidation from the environment. A reductive atmosphere, a surface coating, or a polymeric matrix is commonly employed.15–17
2. Rare earth nanocrystals
A large number and variety of reports on controlled synthesis of rare earth nanostructures have been presented. We attempt to rationally illustrate the typical results classified as zero dimensional (0D), one dimensional (1D), two dimensional (2D), hollow or tubular structures, and other complex situations. Such classification used herein does not correspond to the quantum confinement effects, it is just for the sake of discussion in a clarified sequence. The 0D, 1D, and 2D nanostructures should be defined as nanocrystals with equal or similar sizes in all directions, those with one elongated direction, and those with one flattened direction, respectively.2.1. Zero dimensional nanostructures: nanopolyhedra
Zero dimensional nanocrystals are nanoparticles with spheric shapes and well-facetted nanopolyhedra. Cubes, truncated cubes, truncated octahedra, and octahedra are typical shapes for nanopolyhedra with cubic crystal structures. There have been a great number of reports on various rare earth nanoparticles and only typical results on well-facetted ceria nanoparticles are illustrated below.Ceria-based materials, including CexZr1−xO2, have extensive applications as ultraviolet absorbers, three-way catalysts, and electrolytes in solid oxide fuel cells; ceria is one of the most studied rare earth compounds in recent years. Ceria exhibits a cubic fluorite structure (Fig. 1a). According to a molecular dynamics simulation called “simulated amorphisation and recrystallisation” by Sayle and co-workers, the favoured shape of a ceria nanoparticle is a truncated octahedron, exposing {110} and {111} faces (Fig. 1b).18
 | ||
| Fig. 1 (a) The crystal structure of CeO2; (b) simulated amorphisation and recrystallisation of CeO2nanoparticles (reprinted with permission from ref. 18. Copyright 2004, Royal Society of Chemistry); (c) ceria nanocubes (reprinted with permission from ref. 19. Copyright 2006, American Chemical Society); (d) a ceria octahedral crystallite (reprinted with permission from ref. 20. Copyright 2008, American Chemical Society). | ||
A large number of works have contributed to the control of size and shape of ceria nanocrystals through controlling the crystal growth process.19–25 Ceria nanocubes about 5–10 nm and a well-crystallized octahedron over 100 nm are shown in Fig 1c and d, respectively, as examples. Due to space limitations, the results on ceria are not discussed in detail.
2.2. One dimensional nanostructures: nanorods, nanowires, and nanobelts
One dimensional nanostructures include nanorods, nanowires, and nanobelts. For inorganic compounds, the growth direction of 1D nanostructures is often dominated by the crystallographic symmetry; for example, the growth direction naturally follows the unique axis when the material possesses a trigonal, hexagonal or tetragonal symmetry.4 In addition, synthesis factors such as template, supersaturation, and capping agents are helpful to direct the growth.26Here, examples from rare earth hydroxides, orthophosphates, and orthovanadates are selected to highlight the controlled synthesis of 1D rare earth nanostructures. These rare earth nanostructures show strong dependences on the phase and crystal structure while ion mobility and solubility play key roles, i.e., well-grown 1D nanocrystals are obtained for compounds with 1D crystal structural character when adequate monomers with enough mobility are supplied in the mother liquor. If the solubility is too low, the ions are exhausted before the anisotropic structure is formed and only small nanoparticles are obtained. Adjusting the acidity, or adding chelating reagents to increase mobility of cations, are constructive for these nanocrystals.
General hydrothermal synthesis of rare earth hydroxide nanowires was reported by Wang and Li (Fig. 2).27 Rare earth hydroxide gels are precipitated in basic solution with KOH as precipitator at room temperature and hydrothermally treated in the mother liquor at 180 °C. Sm(OH)3 is taken as an example to disclose the optimal conditions to obtain nanowires. Nanorods with smaller aspect ratios are formed with 5 mol L−1KOH, due to the limited mobility of Sm3+ in concentrated base. With pH values of 6–7, Sm(OH)3 nanosheets are formed instead. When the pH value is between 9 and 10, Sm(OH)3 nanowires with the largest aspect ratio are obtained.
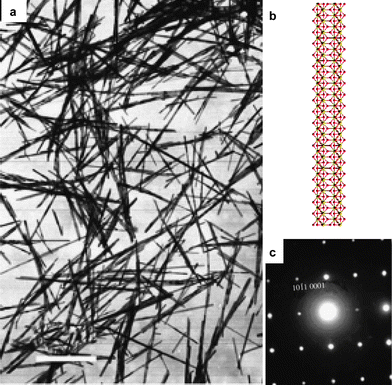 | ||
| Fig. 2 La(OH)3 nanowires: (a) TEM image; (b) crystal model; (c) ED patterns. (a,c) Reprinted with permission from ref. 27. Copyright 2002, Wiley-VCH. | ||
Similarly, strong dependences of morphology and phase on basicity and precipitator are found in the hydrothermal synthesis of ScOOH/Sc(OH)3 nanocrystals.28 With NaOH as precipitator, pH values of 10–14, or with 2.5 mol L−1NaOH, α-ScOOH is obtained, similar to YbOOH obtained by Wang and Li.27 However, when higher concentration of NaOH (5 mol L−1) is used, the product is Sc(OH)3. The aspect ratios of ScOOH nanocrystals decrease with increasing pH values, while Sc(OH)3 forms larger sub-micron crystals. A different precipitator like NH3·H2O gives rise to very different results, and only metastable γ-ScOOH nanoparticles are obtained, due to the weaker mineralization effect of NH3·H2O.
The crystal structure of RE(OH)3 (Fig. 2b) is helpful for an improved understanding of their anisotropic crystal growth. Along the c-axis, there are 1D chains consisting of alternating triple OH− anions and RE3+ cations connected to each other. The 1D chains serve as one intrinsic factor favouring the wire-like morphology.26 With different pH values, OH− ligands can capture or release protons to form O2−/H2O, changing the sign and density of charges on the growing crystal faces to affect the morphology significantly. Therefore, nanosheets are formed with neutral pH values.29
Rare earth orthophosphate 1D nanocrystals are acquired via hydrothermal treatment30–33 and other methods.34,35 The hydrothermal synthesis of light rare earth (La–Eu) orthophosphate nanowires relies on their intrinsic crystal growth behaviour. Rare earth phosphate gel-like precipitates are formed at room temperature, displaying brushy ball-like nanoparticle assemblies.31 Subsequent hydrothermal treatment at 140–240 °C produces nanoparticles/rods/wires of rhabdophane hydrate or monazite dehydrate, depending on temperature and mother liquor acidity.30–32
The hydrothermal temperature is the most important factor that influences the phase of light rare earth orthophosphates. Pure hexagonal products are formed at 140 °C, while complete conversion to a monoclinic phase occurs at elevated hydrothermal temperatures at about 220–240 °C. The hexagonal nanorods/nanowires obtained from hydrothermal synthesis are converted to monoclinic nanorods/nanowires through calcination at 900 °C in air.30
The mother liquor acidity governs the morphology of monazite type rare earth orthophosphates. Syntheses in basic and neutral pH values give rise to particle-like morphology (Fig. 3a), due to the low solubility and mobility of cations. Higher acidity between pH 1–2 allows the nanocrystals to grow and monazite nanowires are formed in the [001] direction (Fig. 3b). Additional H3PO4 leads to smaller aspect ratio particles, due to lowered solubility.31 Similar [001]-grown nanowires of hexagonal phase are obtained with appropriate acidity.32
 | ||
| Fig. 3 Rare earth orthophosphate nanocrystals: (a) monazite LaPO4:Eu nanoparticles (reprinted with permission from ref. 36. Copyright 1999, Wiley-VCH); (b) monazite LaPO4nanowires (reprinted with permission from ref. 31. Copyright 2003, Wiley-VCH) with (c) crystal model of monazite; (d) rhabdophane YPO4 hexagonal rods (reprinted with permission from ref. 35. Copyright 2007, Wiley-VCH) and (e) xenotime (zircon) YPO4nanowires (reprinted with permission from ref. 37. Copyright 2007, American Chemical Society). | ||
The wire-like morphologies of rare earth phosphates are strongly correlated to their crystal structures. For the monazite phase, a rare earth cation and a coordinating phosphate anion are connected through one or two oxygen atoms. The two-oxygen-connected cations and anions are arranged alternately along the c-axis to form a 1D chain, which is energetically favourable for 1D growth in this direction. A similar situation also occurs for hexagonal rhabdophane crystal structures and their nanorods/nanowires.
In contrast to the intrinsic growth behaviour of light rare earth orthophosphates or rare earth hydroxides, the growth of the heavy rare earth orthophosphates is different. The heavy rare earth (Ho–Lu, Y) ions have smaller ionic radii, favouring the tetragonal zircon structure with a smaller coordination number of eight compared with that of nine in a monazite structure. The direct hydrothermal synthesis of heavy rare earth orthophosphate nanocrystals commonly results in xenotime type nanoparticles or brick-like crystallites.
Hexagonal hydrate nanorods are obtained for YPO4 through hydrothermal synthesis mediated by EDTA.33 Subsequent heat treatment at 800 °C allows such nanorods to be converted to zircon type, with the morphology partially collapsing. Regular monodisperse hexagonal YPO4·nH2O nanocrystals are obtained through an oleic acid mediated hydrothermal route at 140 °C (Fig. 3c).35
1D chains of alternating rare earth cations and phosphate anions connected by two O atoms are also found in tetragonal zircon structure, whereas zircon type nanowires are not formed with the simple hydrothermal treatments, probably due to the weak anisotropicity. Cheon and co-workers pointed out that tetragonal crystals show less tendency to form anisotropic nanostructures.4 The zircon type YPO4nanowires are only obtained in oil phase synthesis (Fig. 3d).38 In mixed high-boiling solvents of oleic acid (OA)/oleylamine (OM)/octadecene (ODE) with injection of PO43− species at 180 °C, rare earth phosphate nanocrystals are synthesized.38Monazite type nanopolyhedra for light rare earth orthophosphates and zircon type thin nanowires for medium and heavy rare earth phosphate are obtained. IR spectra confirm the strong interaction between the oleic acid ligands and the nanocrystals.
The hydrothermally obtained monazite nanowires LaPO4:Eu3+ show different photoluminescence spectral features compared with the nanoparticles.36,39 The peak around 620 nm assigned to the 5D0 → 7F2 emission displays stronger intensity, which is related to a different surface-related site for Eu3+ originating from the high anisotropy.
Controlled synthesis of REVO4nanoparticles, nanorods, nanoplates, and other nanostructures have been reported,14,37,40–48 and the typical morphology of small nanoparticles has been studied36,49 Quantum yields of YVO4:Eu3+ nanocrystals are significantly affected by surface defects, and the surface modification with capping agents like citric acid is helpful to control the particle size, to prevent aggregation, and to improve dispersibility. The surface modification is carried out via a post-treatment ligand exchange process or in situhydrothermal synthesis with the ligands.46
The pH is crucial for the synthesis of rare earth orthovanadate nanocrystals. In acidic solvents, VO43− is reversibly condensed into V3O93− and other complex anion groups, and even precipitated as V2O5. Therefore, the aqueous synthesis of YVO4:Eu3+ nanocrystals is usually carried out in basic solutions, whereas concentric alkali leads to hydroxides even in the presence of VO43−.40
Chelating reagents, like EDTA, are helpful for the synthesis of REVO4nanostructures.43–45 Taking CeVO4 as an example, Ce(NO3)3, Na3VO4, and different quantities of EDTA are mixed at room temperature at different pH values from 1–14, and subsequently subjected to hydrothermal treatment at 140–240 °C. By tuning EDTA/RE3+ ratio, temperature, and acidity, various shapes, such as rod-like, flower-like, woollen spheres, hollow spheres, dumbbell-like, particles, and cubes are obtained (Fig. 4c,d). The aspect ratio increases when the acidity decreases, while higher temperature favours cube formation. It is observed in the powder XRD profiles that the relative intensity of the (200) peak over the (112) peak varies significantly with morphology due to the preferential orientations of the powder.
 | ||
| Fig. 4 Rare earth orthovanadates: (a) TEM image and (b) photoluminescence emission spectra of zircon LaVO4:Eu nanorods (profile a) and bulk monazite LaVO4:Eu (profile b) (reprinted with permission from ref. 43. Copyright 2004, American Institute of Physics). CeVO4: (c) hollow nanoparticle assemblies and (d) brick-like crystallites (reprinted with permission from ref. 45. Copyright 2005, American Chemical Society). | ||
The chelating reagent EDTA affects the phase of LaVO4. Without EDTA, monazite type LaVO4:Eu3+ nanoparticles with poor luminescence properties are formed in a direct hydrothermal process, whereas pure zircon type LaVO4 and LaVO4:Eu3+ nanocrystals are synthesized under similar conditions with a small quantity of EDTA to suppress the monazite products. The small monazite nanoparticles are first formed and EDTA facilitates their conversion to larger tetragonal products. Tetragonal LaVO4:Eu3+ is considered as a promising red phosphor similar to YVO4:Eu3+(Fig. 4b).
The nanorods of tetragonal REVO4 (Fig. 4a) obtained through the EDTA mediated routes exhibit a growth direction along the [001] axis, similar to the tetragonal REPO4nanowires.
2.3. Two dimensional nanostructures: nanoplates
Two dimensional nanostructures come with only one confined dimension. 2D nanostructures like quantum wells and epitaxial films have been studied for a long time. The confined direction may arise from the layered structures of materials and the fabrication method such as molecular beam epitaxy, or surface ligands.Hydrothermal syntheses without using surfactants have been carried out for numerous 2D rare earth nanostructures, such as REF3 nanoplates50 and Lu2O3nanosheets with Lu(OH)3 sheets as intermediates.29 These 2D structures synthesized with fine control of pH values and other reaction parameters tend to stack with the main exposed faces.
In contrast, colloidal rare earth nanoplates with uniform shape and size are obtained through precipitation routes in high-boiling solvents or hydrothermal routes in the presence of strong chelating reagents such as oleic acid.
The synthesis in high-boiling complexing solvents is illustrated with rare earth oxide nanoplates. RE2O3 have three phases at ambient conditions: the hexagonal phase, monoclinic phase, and cubic phase. Rare earth oxides are generally synthesized by solid state reactions. The hydrothermal treatment of rare earth oxide powders results in a hydration process to form hydroxides. Thus the synthesis of rare earth oxide nano-materials in the solution phase is only realized in high-boiling solvents at temperatures up to 350 °C.28,51,52
Cao first reported the synthesis of Gd2O3 nanoplates in OA/OM/ODE solvents with the thermolysis of gadolinium acetate.51 The systematic synthesis of rare earth oxide nanoplate/nanopolyhedron series is fulfilled via the thermal decomposition of rare earth benzoylacetonate (BA), acetylacetonate (acac), and acetate (ac) precursors.24,28,52
A typical synthesis process in high-boiling solvent OA/OM/ODE starts with producing the precursor for pyrolysis. For instance, rare earth benzoylacetonate hydrate RE(BA)3(H2O)n is synthesized by mixing rare earth nitrate aqueous solution and 1-benzoylacetone ethanol solution together under stirring at pH 6–7. Then, a small amount of RE(BA)3(H2O)n is added to OA/OM at room temperature. After bubbling with Ar, the low-boiling point fractions including water are removed under vacuum at about 100 °C. The precursor solution is then heated to 250–330 °C for a certain time under Ar. After the solution has cooled naturally, ethanol is poured into the solution to precipitate the products. The as-precipitated nanocrystals are dispersible in non-polar solvents such as hexane, toluene, and cyclohexane. Fine-tuning of the polarity of the mixed solvent and the concentration of the nanocrystals allows the fabrication of a micron scaled superlattice on a TEM grid. The nanocrystals obtained through such a method form a new family of materials featuring high crystallization, uniform size and shape, and self-assembly behaviour.53–55
The capping agents OA and OM impose strong effects on both the phases and the morphologies of the nanocrystals. For all rare earths except CeO2, RE2O3 nanoplates in cubic phase are formed (Fig. 5), as determined from the XRD patterns. For ceria, nanopolyhedra are formed. The cubic phase is not a common product under ambient conditions for light and medium rare earth oxides, therefore, the phase is controlled and stabilized by these capping agents.
 | ||
| Fig. 5 Rare earth oxide nanoplates: (a) Pr2O3; (b) Eu2O3 (reprinted with permission from ref. 52. Copyright 2007, American Chemical Society). | ||
Similar synthesis can be applied to other rare earth compounds. Rare earth fluoride nanocrystals are obtained from aqueous solution by fine control,56,57 while high-quality monodisperse rare earth fluoride nanoplates are synthesized through a general route by the thermolysis of RE(CF3COO)3 precursors in OA/ODE.58,59 Composition of the mixed solvents, reaction temperature, and reaction time are finely controlled to obtain rare earth fluoride nanoplates of different sizes and shapes, or to obtain rare earth oxyfluoride nanoparticles. The shape of REF3 nanoplates depends on the phase, cationic radii, and synthesis parameters. Light rare earth fluorides in a trigonal phase form nanoplates in trigonal, truncated trigonal, and hexagonal shapes, enclosed by {110} side faces and {001} top faces (Fig. 6a). Large cations like La3+ favour trigonal shapes while small cations like Sm3+ favour hexagonal shapes. For LaF3, short reaction times lead to trigonal shapes while longer times lead to truncated shapes, and finally hexagonal shapes are formed. Such evolution is ascribed to Ostwald ripening. The heavy rare earth fluorides in an orthorhombic phase exhibited parallelogram shaped plates enclosed by {101} and {10−1} side faces and {010} top faces (Fig. 6b). There is a topotactic relation between the structures of the trigonal phase and the orthorhombic phase, in which the c-axis in the trigonal phase corresponds to the b-axis in the orthorhombic phase (Fig. 6c). Such a relation reveals that the {001} face of trigonal REF3 is the equivalent of the {010} face of the orthorhombic phase. OA ligands attach on such faces and impose a decisive effect on the formation of this 2D morphology (Fig. 6c).58,59 If the experimental conditions are tuned, for example, by adding OM or heating to higher temperature, monodisperse REOF nanoparticles could be obtained (Fig. 6d).53
 | ||
| Fig. 6 (a) LaF3 nanoplates (reprinted with permission from ref. 58. Copyright 2006, American Chemical Society); (b) GdF3 nanoplates; (c) schematic illustration of relation between trigonal and orthorhombic phase of REF3 and their nanoplates (b and c are reprinted with permission from ref. 59. Copyright 2006, Wiley-VCH); (d) LaOF:Eu nanoparticles (reprinted with permission from ref. 53. Copyright 2008, American Chemical Society). | ||
RE2O2S exhibits a trigonal crystal structure, in which RE3+ is coordinated by three S2− and four O2−. Such a structure is considered as S2− layers alternating with layers composed of RE3+ and O2−. Gao and co-workers reported the synthesis of monodisperse EuS or Eu2O2S nanocrystalsvia the thermolysis of a single source molecular precursor Eu(phen)(ddtc)3 (ddtc = dialkyldithiocarbamate) in high-boiling solvent system,15,60,61 in which ddtc is a ligand commonly used to synthesize sulfides through thermolysis routes. When the precursor decomposes under the protection of N2 flow in OM, monodisperse EuS nanoparticles are obtained below 260 °C, while flower-like nanoparticles are assembled above 260 °C. In the absence of protective conditions, short Eu2O2S nanorods are formed in OM solvent. If OA/OM/ODE solvents are used, Eu2O2S nanoplates are formed. Obviously, OA is a stronger coordinating reagent compared with OM and the stronger interaction between OA and nanocrystals is responsible for stabilizing the plate-like morphology.
The shapes of such 2D structures are in accordance with the symmetry of the projected crystal face, i.e., square for the (001) face in cubic RE2O3, round/semi-hexagonal for (111) in cubic RE2O3, trigonal, truncated-hexagonal, or hexagonal shapes for (001) in trigonal REF3, and parallelogram or zigzagg shapes for (010) in orthorhombic REF3.
The strong anisotropic nature of these nanocrystals is observed through XRD patterns. While the structures of RE2O3 nanoplates are determined by XRD patterns to be cubic, their morphologies normally exhibited symmetry breakdowns as square or round (semi-hexagonal) plates. Then the confined crystal face is widened in the XRD patterns but non-confined equivalents are not, i.e., superimposed peaks with narrow and wide shapes are observed at the same position.
Sometimes, the thickness of the 2D nanostructures might be as thick as 1.5–3 nm, i.e., only a few layers of atoms/ions should be counted in this direction. For example, one hexagonal SmF3 nanoplate sample is measured with the HRTEM images to be about 1.4–1.8 nm.59 Obviously, the different thicknesses of these nanoplates are related to the crystal structure. Trigonal SmF3 shows a cell of a = b = 0.6952 nm, c = 0.7122 nm as shown in a PDF card (JCPDS 12-792), and there are two layers of Sm3+ coordinated by F− in one cell perpendicular to the c-axis. Therefore, the spacing between the cation layers is about 0.36 nm and the above-mentioned SmF3 nanoplates contain about 4–5 layers of cations.
The confining on plate faces can be figured out in experiments with an attempt to grow a shell for LaF3:Eu nanoplates. When a second growth of LaF3 is added to the LaF3:Eu trigonal nanoplates, the thickness of the plate increases only by 0.1 nm while the sides increase by 3.3 nm.59 In fact, the increase in thickness is less than one atom layer and it is just filling of defect sites. Such a growth mode is a “framed” style, that is, only the side sizes are increased.
Another similar strategy utilizing the strong confining ability of oleic acid is the hydrothermal method. Li and co-workers prepared doped zircon type LaVO4nanocrystals through an OA assisted hydrothermal route. Uniform square nanoplates with sides of about 40 nm and thickness of about 10 nm are obtained. The c-axis is the confined direction.62 Very similar results are obtained through the synthesis in high boiling solvents OA/ODE.48 YBO3 lens-shaped microparticles are prepared through a solvothermal synthesis in aqueous ethanolic solution mediated by oleic acid.63
2.4. Tubular and hollow nanostructures
Nanotubes and hollow structures are different from nanowires or nanoplates in topology. If some crystal faces are confined and other crystal faces are allowed to grow, these tubular, porous, or hollow structures will not be formed directly. Therefore, more complex mechanisms for these structures are proposed.Tubular structures of many types of materials such as carbon, metals, and metal oxides have become well known in the last decade. The tubular nanostructures can be classified into thin-wall tubes, typically related to compounds with two dimensional structures, and thick-wall tubes, related to three-dimensional compounds.64 Mechanisms and methods such as scrolling of nanosheets, etching of terminal faces, hard or soft templates effects, and Kirkendall effects for nanorods are suggested for the formation of monocrystalline and polycrystalline nanotubes.1,5 Various rare earth nanotubes have been reported in recent years.
The productive synthesis of rare earth hydroxide/oxide nanotubes is first accomplished with mediation of appropriate surfactants in aqueous solution. Yada and co-workers reported a general soft template strategy utilizing sodium dodecylsulfate as surfactant.69 Rare earth nitrates or chlorides, urea, sodium dodecylsulfate, and water are mixed at 40 °C to form a transparent solution and heated at 80 °C for 100 h. The hydrolysis of urea raises the pH values and precipitates rare earth hydroxides. Anion exchange with sodium acetate is carried out to remove the surfactants. Rare earth oxide nanotubes with small inner diameters of 3 nm and wall thickness of 1 nm are finally synthesized after calcination (Fig. 7a).69 Synthesized Y2O3:Eu3+ nanotubes exhibit different spectral characteristics from those of nanoparticles or bulk materials.70Hydrothermal synthesis at 120–180 °C has also been reported, with polyethylene glycol (PEG), methyl methacrylate (MMA), sodium dodecyl benzenesulfonate (SDBS), or CTAB to fabricate rare earth hydroxide nanotubes. The hydroxide nanotubes are subsequently dehydrated to obtain rare earth oxide nanotubesviacalcination.71–74
 | ||
| Fig. 7 Rare earth nanotubes: (a) erbium-based nanotubes (reprinted with permission from ref. 69. Copyright 2002, Wiley-VCH); (b,c) Dy(OH)3 and Dy2O3nanotubes (reprinted with permission from ref. 75. Copyright 2003, American Chemical Society); (d) crystal model of RE(OH)3; (e) atomistic simulation of ceria nanotubes (reprinted with permission from ref. 78. Copyright 2007, American Chemical Society); (f) NaYF4nanotubes (reprinted with permission from ref. 79. Copyright 2007, Wiley-VCH). | ||
Later, synthesis of rare earth hydroxide/oxide nanotubes in the absence of surfactants was also reported. Yada and co-workers fabricated nanotubes and nanotube hierarchical assemblies through a homogeneous precipitation by urea hydrolysis at 80 °C.64 Xu and co-workers reported the hydrothermal synthesis of monocrystalline dysprosium and terbium hydroxide nanotubes from rare earth oxide powder or colloidal hydroxide precipitation at 120–160 °C (Fig. 7b,c).75 Other rare earth hydroxide/oxide nanotubes including CeO2 and Y2O3:Er have also been reported.71,76,77 Generally, lower temperature with higher basicity, or higher temperature with lower basicity, may be conducive for the formation of rare earth hydroxide nanotubes.
The diameters of these rare earth nanotubes vary from a few nanometres to sub-micrometre size, depending on the different synthesis conditions. The length of these nanotubes often reaches hundreds of nanometres or up to several micrometres. Generally, nanotubes obtained with surfactants have larger inner diameters relative to the total size, with some surfactants incorporated into the structure, while those prepared without surfactants are better crystallized. Some of the obtained nanotubes exhibit interesting self-assembly behaviour to form spherical morphologies.69,75
The growth directions of RE(OH)3 nanotubes are reported to be [001] or [100], both reasonable in view of the crystal structures since the former is the same growth direction as the rare earth hydroxide nanowires, while the latter might hint at the curling of the (001) face, like the fullerene-like nanoparticles discussed in the next section. The axis directions of RE2O3 or CeO2nanotubes that are obtained by dehydration from hydroxides vary. The different growth directions assigned to RE(OH)3 nanotubes should be further analysed taking into consideration the different synthesis conditions and the crystal structures.
Atomistic simulation of ceria nanotubes by Parker and co-workers based on the Born model revealed that in multilayer wrapped ceria nanotubes, polycrystalline behaviour like boundaries, junctions, and dislocations are important to accommodate the strain (Fig. 7e).78
Wu and co-workers reported tubular NaSmF4 and NaHoF4 crystallites through hydrothermal synthesis at 140 °C and pH = 3 in the presence of EDTA.80 Single-crystalline rod-in-tube nanocrystals are also observed.55 Similar single-crystalline tube-in-tube morphology could be found in α-Fe2O3nanocrystals synthesized in acidic solution with PO43− or SO42−,81 where the etching of spindle-like nanocrystals is supposed to be one key step.
Another example of NaREF4nanotube is reported by Zhao and co-workers.79 They obtained uniform NaREF4nanostructure arrays with multicolour up-conversion properties through an oleic acid assisted hydrothermal method. The nanostructures could be nanotubes, nanorods, and flower-patterned nanodisks. The uniform hexagonal nanotubes form honeycomb-like assemblies (Fig. 7f). The effects of F− and OA/NaOH are discussed. With more F−nanorods tend to form, while with more OA/NaOH patterned nanodisks tend to form. Such a result suggests an etching process by the OA surfactants on the (0001) surface.
Hollow nanoparticles of rare earth fluoride and hydroxide are obtained through hydrothermal routes.67,82 REF3 and RE(OH)3 hollow nanoparticles contain single or multiple cavities. Around the cavity, lattice fringes, which meet the separation between (002) faces for fluorides or (001) faces for hydroxides, are observed.
Hollow nanoparticles of MoS2 are similar to fullerene and are formed with layers of S atoms held together by van der Waals forces. REF3 and RE(OH)3 are typical ionic compounds without weakly interacting layers, but obvious cleavages in the above mentioned directions are also helpful to stabilize the cavities. Further studies are required both to clarify the mechanism and to find applications for similar hollow rare earth nanoparticles.
3. Controlled synthesis
The controlled synthesis of rare earth nanostructures depends on both utilization of the intrinsic structure via phase control, and extrinsic means such as the manipulation of nucleation and growth process and post-treatments. In addition, there are still many composite and complex nanostructures for rare earth nanomaterials, which will not be covered in detail herein due to space limitations.3.1. Phase control
Since the correlation of crystal structure with the morphology is strong for rare earth nanocrystals, the control of phase is powerful in tuning the morphology.NaYF4 is one of the best hosts for up-conversion luminescence. Up-conversion photoluminescent materials with applications as bio-labels for good penetration of infrared light through living bodies attract great research interest.83 The multiple photon process of up-conversion emission is very sensitive to the lattice host in intensity, efficiency, and chromaticity. Colloidal up-conversion nanoparticles of rare earth phosphates, oxides, and oxysulfides have been reported.84,85 Up to now, the best up-conversion material reported is still β-NaYF4:Yb,Er, whose nanocrystals have been subjected to extensive studies.34,49,86–90
There exist two NaREF4 polymorphs, cubic α-NaREF4 and hexagonal β-NaREF4, and the latter include excellent up-conversion hosts. Hence, the phase control of NaREF4 is of significant interest.
High quality NaREF4nanocrystals are obtained in OA/OM/ODE solvents with controlled size, chemical composition, and surface state. Both monodisperse cubic and hexagonal NaREF4nanocrystals are acquired(Fig. 8).34,88 The Na/RE ratios, solvent compositions, reaction temperatures and times are governing parameters, and the cationic radii of RE3+ also lead to different crystal growth and phase transition behaviours.
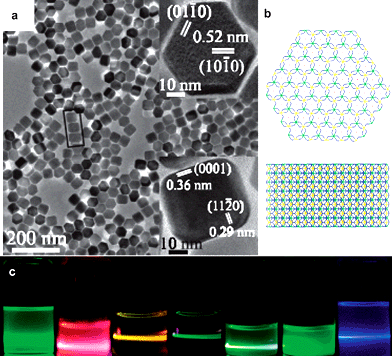 | ||
| Fig. 8 (a) NaHoF4nanocrystals (reprinted with permission from ref. 88. Copyright 2006, American Chemical Society); (b) crystal structure of β-NaREF4; (c) multi-colour fluorescence up-conversion photographs of doped NaYF4nanocrystals (reprinted with permission from ref. 34. Copyright 2007, American Chemical Society). | ||
The bulk materials of α-NaREF4 are not stable at room temperature and can be transformed to a variety of products when annealed. However, α-NaREF4nanoparticles seem to be stabilized by their small sizes and remain unchanged at room temperature for a long time. Pure α-NaREF4nanoparticles can be formed with relatively low temperature, short time, and small Na/RE ratios.
In contrast, β-NaREF4 is formed under more drastic conditions, i.e., high temperature, long time and large Na/RE ratios. An α-NaREF4 crystallite precursor route with injection of additional Na(CF3COO) at high temperature has also been investigated for the synthesis of β-NaREF4nanocrystals. The transformation of the α to the β phase occurs both in the direct synthesis route and the α-NaREF4 crystallite precursor route. Such transformation varies with the lanthanide series, which is thus divided into 3 groups (I: Pr and Nd; II: Sm to Tb; III: Dy to Lu, Y). The second group of compounds of β-NaREF4 are formed more easily, in agreement with the report by Haase and co-workers that β-NaGdF4 is obtained more easily than β-NaYF4.91 For the first and third groups including NaYF4, it is harder to prepare monodisperse high quality β-NaREF4nanocrystals, and the α-NaREF4 crystallite precursor route is proposed.
The mechanisms in the nucleation and crystalline growth processes are of great importance for the development and rational design of these processes. Up-conversion spectra can be applied to monitor the formation of luminescent species like NaYF4:Yb,Tm and NaYF4:Yb,Er nanocrystals in the OA/OM/ODE solvent system with temporal evolution.90 As the characters of up-conversion emission including total intensity and ratio of green to red emission intensities are related to the nucleation, phase transition, and crystallite size, this enables the different growth stages and mechanisms of phase transition to be distinguished by monitoring the up-conversion spectrain situ or ex situ.
So long as the phase of NaREF4 is well controlled, the intrinsic shape of these nanocrystals is also determined. Cubic α-NaREF4 always exhibits particle-like morphology while hexagonal β-NaREF4 usually displays trigonal symmetry with rod-like or plate-like morphologies, depending on the tunable aspect ratios.
3.2. Nucleation and growth process control
The nucleation and growth processes for rare earth nanocrystals are fine aspects for their formation compared with the effect of intrinsic structures.Vaterite type YBO3:Eu3+ exhibits high optical damage threshold and strong VUV absorption properties, which are suitable for applications in plasma display panels and Hg-free fluorescent lamps. Previous studies suggested BO33−, instead of other borates, is important for VUV absorption. YBO3:Eu3+ emits red-orange lights under UV excitation, due to higher symmetric sites of Eu3+, partially forbidding the electric dipole transition of 5D0 → 7F2 emission according to the Judd–Ofelt theory.92 The fluorescence intensity and chromaticity are significantly tuned in nano-sized YBO3:Eu3+ for its higher disorder status.92–94
Donut-like YBO3:Eu3+ nanoparticle assemblies are synthesized in nitrate solution at 180 °C and pH 8.94 The crystalline size increases significantly after calcination while the shape of the assembly retains. In contrast, hexagonal YBO3:Eu3+ micro-plates with the size of 1 μm in diameter and 0.5 μm in thickness are prepared under similar conditions by hydrothermal treatment at 180 °C and pH 7 with high concentration of NH4Ac as both a coordination reagent to adjust the morphology and a buffering reagent to control the pH values during the reaction (Fig. 9).93
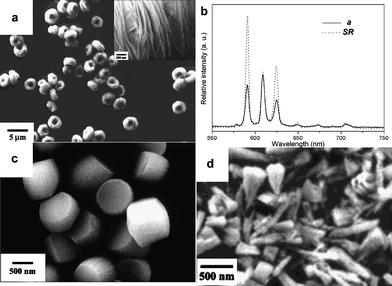 | ||
| Fig. 9 YBO3:Eu nanostructures and photoluminescent spectra. (a,b): Reprinted with permission from ref. 94. Copyright 2004, American Chemical Society. (c,d): Reprinted with permission from ref. 93. Copyright 2004, American Chemical Society. | ||
Subtle differences in the experimental conditions caused very different results. The milky precipitation of Y(OH)3 at pH 8 served as seeds for the subsequent crystal growth and affected the nucleation process, therefore, the donut-like nanosheet assembly could be formed. The NH4Ac not only served as a buffer to control pH values, but also served as a capping agent to mediate the synthesis. For rare earth borates, which are usually difficult to crystallized, proper control of nucleation and growth process would effectively produce various well-defined nanostructures.
3.3. Morphology-maintained conversion
Some rare earth compound nanostructures can be obtained by the direct conversion from the established counterparts without destroying the morphology, via solution-based reactions or solid state reactions. Such a strategy will not only provide more access to useful nanostructures, but also put new avenues forth in overcoming the restriction of the intrinsic symmetry of the targeted compounds.Rare earth hydroxides are versatile as intermediates for various rare earth anisotropic nanostructures, such as oxides, oxyfluorides, and oxysulfides, by employing morphology-maintained conversions.67 The dehydration of well-crystallized RE(OH)3 nanowires at 500 °C leads to the formation of RE2O3nanowires; calcination under sulfur atmosphere at 750 °C produces rare earth oxysulfide nanowires; while hydrothermal treatment at 120 °C in the presence of F− results in rare earth oxyfluoride nanowires.27,67
The synthesis of EuO nanorods is a typical conversion example. EuO with a cubic rock salt structure is a ferromagnetic semiconductor with a bandgap of 1.1 eV and bulk Curie temperature (Tc) of 69 K. Bierman and co-workers obtained EuO nanorodsvia a three-step synthesis route. Eu(OH)3 is first precipitated from Eu(NO3)3 by hexamethylenetetramine (HMT) at 95 °C, excluding Na+ or K+. Then the precipitates are calcined at 800 °C to obtain Eu2O3 powder samples, followed by annealing in Eu metal atmosphere to reduce the materials to EuO. As both EuO and Eu2O3 exhibit cubic symmetry, the obtained polycrystalline nanorod morphology is ascribed to be formed from the Eu(OH)3 precursor.16
Wu and co-workers reported a boron–sulfur shape-preserving conversion of rare earth hydroxides to obtain RE2O2S, RE2O2S2, and RES2 (RE = La, Nd) nanowires. The sulfidation process is well controlled with time, temperature, and RE : S loading ratios. RE2O2S or RE2O2S2 is obtained with a partial sulfidation in 10 min at 500 °C and RES2 is obtained with a complete sulfidation in 24 h at 400 °C.95 A crystallographic relation between the precursors and the products is not observed and a self-sacrificing templating mechanism is proposed.
In order to fabricate nanostructures of ceria other than the cubic class morphologies, extrinsic conditions breaking the cubic symmetry have to be applied. In aqueous routes, Ce(OH)3 is easily precipitated under basic conditions and is quickly oxidized into Ce(IV) under air. Therefore, rod-like and tubular structured ceria are often obtained through hexagonal Ce(OH)3 intermediates with the same shape, sometimes in a one-pot process. For example, ceria nanorods of 10 nm in width and 50–200 nm in length can be directly obtained, by virtue of the temporary formation of hexagonal Ce(OH)3, through a hydrothermal treatment at 100 °C with over 6 mol L−1NaOH. The obtained ceria nanorods are single crystalline in nature with a growth direction of [110].96
A composite-hydroxide-mediated (CHM) approach proposed by Wang and co-workers is available to obtain single crystal complex oxide nanostructures.97 Highly crystallized and well-defined La(OH)3 and La2O3 nanobelts are obtained through the CHM method, where a eutectic mixture of NaOH and KOH with an optimal melting point at 165 °C is used as solvent. LaAc3 with a small amount of water is added and then the mixture is heated to 200 °C, yielding single crystalline La(OH)3 nanobelts with a growth direction of [110]. After calcination, single crystalline La2O3 nanobelts in hexagonal phase retaining the original shape are achieved with a growth direction of [010].98 This method is also applied to prepare doped ceria nanowires, in which Ba2+ can hardly be doped in normal aqueous routes due to the poor solubility of Ba(OH)2 in water.99
Besides hydroxides precursors, conversion from the other precursors is also reported. Hydrothermal treatment and follow-up calcination of YBO3:Eu3+ nanosheet assemblies in Na3VO4 solution result in YVO4:Eu3+/YBO3:Eu3+ composites, which combine the high luminescence intensity and excellent chromaticity for YVO4:Eu3+ and good VUV absorption performance for YBO3.100
3.4. Composite and complex structures
In addition to the simple 0D, 1D, and 2D nanocrystal building blocks, other more complex nanostructures like core/shell structures, composites, branched structures, mesostructures, etc., are also fabricated with great research interest. However, owing to space limitations, we only illustrate a few examples.Core/shell structures have been extensively constructed for nanoparticles to protect and modify the surface, build quantum wells, provide multi-functionality, or enhance luminescence properties.14,17,83,101 Monodisperse silica coated nanoparticles ready for photonic crystals fabricated via controlled hydrolysis of tetraethyl orthosilicate (TEOS) are shown in Fig. 10a.101
 | ||
| Fig. 10 More complicated rare earth nanostructures: (a) monodisperse Tb(OH)3@SiO2 colloidal sphere capable of forming photonic crystals (reprinted with permission from ref. 101. Copyright 2006, Wiley-VCH); (b) single CeO2nanowire (1) and Pt nanocrystal/CeO2nanowire (2) (reprinted with permission from ref. 103. Copyright 2008, American Chemical Society); (c) ceria nanoflowers (reprinted with permission from ref. 104. Copyright 2008, Wiley-VCH); (d) ceria–zirconia ordered mesoporous superstructures (reprinted with permission from ref. 105. Copyright 2008, American Chemical Society). | ||
Depositing noble metal nanoparticles like Au and Pt on ceria nanocrystals greatly improves their catalytic performance, for instance, in water–gas shift catalysts.102 Liao and co-workers demonstrated a single ceria nanowire deposited with Pt nanoparticles as a well designed gas sensor nanodevice to selectively detect CO gas (Fig. 10b).103
Ceria nanoparticles can assemble and fuse in different dimensions by oriented attachment mechanisms to form flower-like nanocrystals under fine control. Ceria nanoflowers are obtained by thermal decomposition of (NH4)2Ce(NO3)6 in OA/OM solvents at 230–300 °C. The reaction is monitored through in situ electrical resistance measurements and the conductive species are deduced to be NO3− ions. A sharp increase of resistance is observed in the range 230–250 °C, accompanied by a violent reaction. TEM observations proved that ceria nanoflowers are formed at the same time. Therefore, the sudden consumption of free NO3− ions is involved in the formation of flower-like nanostructures. In Fig. 10c, the flower-like morphology of ceria is shown, which is made up of truncated octahedra attached to each other mainly by {111} facets.104
Mesostructured materials are of particular interest in catalytic applications. Sol–gel processes with structure directing agents are well developed to assemble highly ordered mesoporous structures of non-silicate oxides.6 Recent developments on the preparation of rare earth mesostructures involved not only the assembly of pre-synthesized nanocrystals but also the sol–gel process. As an example, ordered mesoporous ceria–zirconia solid solutions are synthesized based on a sol–gel process combined with evaporation-induced self-assembly in ethanol with block copolymer P123 as the template (Fig. 10d).105
4. Fabrication and simulation of nanocrystal superstructures
The “bottom-up” strategy is essential in nanotechnology, where small building blocks such as atoms, molecules, and differently shaped and sized nanocrystals are organized into applicable forms like superlattices, films, and devices.106,107 Colloidal nanocrystals capped by surfactants or coated by silica could self-assemble into ordered superstructures. Such superstructures can modulate the properties of individual nanocrystals and give rise to novel collective properties.108A range of methodologies, for example, the Langmuir–Blodget method,109polymer film templating,110 and evaporation-induced self-assembly106 have been proposed to fabricate nanocrystal superlattices with short-range or long-range ordering. Furthermore, size controlled synthesis and size separation techniques111–114 are applied to obtain nanocrystals with narrow size distribution and uniform shape as qualified building blocks.
The structural variety of nanocrystal superstructures has been screened in semiconductors, metals, and metal oxides by Murray and co-workers.106,115 They fabricated more than 13 types of binary superlattices with spherical nanocrystals as building blocks. Furthermore, they also demonstrated possible structural diversity when non-spherical nanocrystals, such as nanowires, nanoplates, and nanotubes, are introduced.106
Generally, there are several important characteristics in the process of self-assembly. The formation of superlattices strongly depends on the size uniformity of nanocrystals. In addition, the spacing between these nanocrystals is well matched to the length of the attached ligands. This suggests that the van der Waals interactions between carbon chains are crucial for the formation of superlattices. As a result, the structures of superlattices are more related to the shape and size of nanocrystals, rather than to the nature of nanocrystals.
For rare earth compounds, there are a number of examples of differently shaped monodisperse nanocrystals forming diverse orderly aligned superstructures.35,37,52,58–62 For instance, Fig. 11 shows a gallery of superstructures ranging from necklace-like ceria assemblies with poly(vinylpyrrolidone) (PVP, Fig. 11a),116 LaPO4:Eu polyhedra superlattice (Fig. 11b), to SmF3 hexagonal nanoplates forming a wheel and axis (Fig. 11c) or “face-to-face” superlattice (Fig. 11d).
 | ||
| Fig. 11 Superstructures based on rare earth nanocrystals: (a) pearl necklace-like ceria nanoparticle assemblies (reprinted with permission from ref. 116. Copyright 2006, American Chemical Society); (b) LaPO4:Eu nanopolyhedra superlattice (reprinted with permission from ref. 37. Copyright 2007, American Chemical Society); (c,d) superstructure of SmF3 nanoplates (reprinted with permission from ref. 59. Copyright 2007, Wiley-VCH). | ||
Despite the large variety of nanocrystal superlattices, their geometries and formation mechanisms are currently considered with simple models, such as nanocrystals covered with exchangeable organic ligands, and assembly driven by steric effects. However, the exact shape of such brushy nanocrystals is not clear, and the assembly behaviour is usually only understood on instinct.
We carried out simulation work in order to clarify the mechanisms and explore the structural possibilities in self-assembly of nanocrystal structures. A colloidal nanocrystal is modelled as a box-like semi-rigid lattice fully covered with non-breaking-off dangling organic ligands. A simple coarse-grained model is built based on the potential field for lipids developed by Marrink and co-workers.117 Each “single atom” in such a coarse-grained model represents 2 to 4 atoms in the ligands, while for the core lattice representing the nanoparticle, the “atom” just represents the site linking to the ligands. The carbon chains of organic ligands are set to be “apolar” in the potential field, attracting each other slightly at the normal distance to mimic the van der Waals interactions. A very fast free molecular simulation package, GROMACS, is used for molecular dynamics simulation herein.118 Based on this model, we can visualize the assembly process of these differently shaped colloidal nanocrystals (Fig. 12). Though the coarse-grained model ignores many details of the nanocrystals and ligands, it still presents much information at the mesoscale. The model is simple and effective. It allows the motion of tens of nanoparticles to be simulated with a personal computer, and can be well utilized in most chemical labs.
 | ||
| Fig. 12 Molecular dynamics simulation based on a coarse-grained model of oleic acid capped nanocrystals. The nanoparticles are in blue. (a) A single nanocube; (b) a single bent nanoplate; (c) self-assembly of nanocubes; (d) self-assembly of nanowires. | ||
There are some interesting findings from our simulation. If the attached points for organic ligands are not at suitable distances, the carbon chains of the ligands might pull or push each other to induce some strain for the nanoplates or nanowires, which favours bent or curved formations, especially when the nanoplates or nanowires are very thin or in the formation process (Fig. 12b). The experimentally observed ring-like Gd2O3nanostructures seem to be good proof of this.119 When the size of nanoparticles is smaller than the length of ligands, the ligands can not well cover the particles because they tend to aggregate in certain directions, and the particles are thus pulled together, which might assist the possible oriented attachment process.
The small 0D nanoparticles covered with soft and attracting organic layers are easy to assemble into ordered close-packed superlattices (Fig. 12c). However, considering the re-orientation barrier for anisotropic nanowires, the organic layers sticking to each other hinder the ordering greatly. As a result, a perfect assembly of anisotropic nanowires is not completed within the same simulation time needed for that of 0D nanoparticles (Fig. 12d).
As a primary simulation, it sheds light on the assembly process of small nanocrystals without long-range interactions. Further study is necessary to provide insight into the assembly behaviour of even larger and more complex systems, facilitating the construction of new superstructures.
5. Remarks and perspective
In the past years, controlled synthesis of rare earth nanostructures has been extensively explored. Based on the intrinsic crystal structures, fine-tuning through reliable and versatile solution-based routes is productive for high-quality, well-controlled, shape- and size-uniform rare earth nanostructures. These functional rare earth nanomaterials exhibit excellent stability and tailorability and are readily available as 0D, 1D, and 2D nano-building blocks for novel nanodevices for optics, sensor, and bio-applications.In addition to the rare earth nanostructures discussed above, there are also abundant studies on nanosized rare earth ion doped materials, rare earth based complex oxides, and rare earth metals and alloys, etc. Such attempts to form novel rare earth nanomaterials are continuing. Mallah and co-workers reported the fabrication of rare earth luminescent coordination nanoparticles, which shed new light on rare earth luminescent materials by connecting the advantage of avoiding detrimental concentration quenching with tunable spacing ligands and the premium water solubility and stability provided by PVP coverage.120,121 Coordination nanoparticles and other rare earth nanomaterials with organic components will unquestionably enrich the rare earth functional nanomaterials.
Current synthesis strategies depend highly on trial-and-error approaches instead of on rational systematic designs.4 In contrast, new methodologies for synthesizing functional rare earth nanocrystals should meet special application-based requirements with a pre-designed synthesis route. Further demands for success in this field include environment-friendly, scalability, versatility, and reliability to obtain nanocrystals with precisely controlled shape and size, desired surface states, and dispersibility in water or low polar solvents. The optical, magnetic, or catalytic properties of rare earth nanomaterials should be enhanced and well utilized in this way.
For designing rational synthesis strategies, the mechanism of reaction process should be well clarified. The development of in situ monitoring techniques, such as optical and electrical measurements,90,104 is of great urgency. Such development can open up opportunities of using these powerful tools to reveal the underlying mechanisms in the crystal nucleation and growth processes. Another way for better understanding the guiding principles of nanocrystal growth could be pursued by simulation work.122 The simulation of crystal growth behaviour, surface effect induced phase, morphology and property control, and assembly of rare earth nanocrystals would be essential topics for understanding the structural characteristics, formation mechanisms, and shape control factors.
When advanced synthesis methodologies are developed, functional rare earth nanocrystals with controlled phase, composition, size, shape, surface, and certain exposed faces can be obtained to serve as excellent building blocks, which are expected to be integrated into novel nanodevices to exhibit both the properties of individual nanocrystals and collective properties, for example, catalytically active hierarchical ceria nanocube assemblies. The unique luminescence properties of rare earth nanocrystals assure their potential applications in optical devices. While further developments of various superstructures reveal new interesting properties and applications of rare earth nanomaterials, the underlying photophysics, including the energy transfer processes within and between luminescent nanocrystals, emission spectra related surface distortion and point symmetry should be further elucidated. Rare earth superlattice nanodevices will be able to provide interesting modulated optical properties and applications. Novel superlattices could be fabricated in three dimensions on a large scale and with new structural varieties. Multifunctional nanostructures/nanodevices composed of rare earth nanocrystals and other nanocrystals could also be designed in diversified ways, for example, different components could be linked together in a binary or more complex superlattice or through well-defined hierarchical nanostructures. Such devices may be utilized as sensors and magnetic or optical devices.
In summary, rare earth nanomaterials are a promising class of novel advanced materials for various applications,13,123 however, great challenges for practical applications are still pending for investigators.
Acknowledgements
This work is supported by the NSFC (Grant Nos. 20671005, 20221101, and 20423005) and MOST (Grant No. 2006CB601104).References
- R. H. Baughman, A. A. Zakhidov and W. A. de Heer, Science, 2002, 297, 787 CrossRef CAS.
- W. L. Barnes, A. Dereux and T. W. Ebbesen, Nature, 2003, 424, 824 CrossRef CAS.
- Y. Yin and A. P. Alivisatos, Nature, 2005, 437, 664 CrossRef CAS.
- Y. W. Jun, J. S. Choi and J. Cheon, Angew. Chem., Int. Ed., 2006, 45, 3414 CrossRef CAS.
- R. Tenne, Nat. Nanotechnol., 2006, 1, 103 Search PubMed.
- S. W. Boettcher, J. Fan, C. K. Tsung, Q. H. Shi and G. D. Stucky, Acc. Chem. Res., 2007, 40, 784 CrossRef CAS.
- R. M. Ormerod, Chem. Soc. Rev., 2003, 32, 17 RSC.
- L. H. Slooff, A. van Blaaderen, A. Polman, G. A. Hebbink, S. I. Klink, F. C. J. M. van Veggel, D. N. Reinhoudt and J. W. Hofstraat, J. Appl. Phys., 2002, 91, 3955 CrossRef CAS.
- J. Kaspar, P. Fornasiero and M. Graziani, Catal. Today, 1999, 50, 285 CrossRef CAS.
- A. Trovarelli, Catal. Rev. Sci. Eng., 1996, 38, 439 CrossRef CAS.
- J. F. Herbst, Rev. Mod. Phys., 1991, 63, 819 CrossRef CAS.
- J. G. Bednorz and K. A. Muller, Z. Phys. B: Condens. Matter, 1986, 64, 189 CAS.
- G. K. Liu and X. Y. Chen, Handbook on the Physics and Chemistry of Rare Earths, Spectroscopic Properties of Lanthanides in Nanomaterials, Elsevier, Amsterdam, 2007, vol. 37, p. 99 Search PubMed.
- V. Buissette, D. Giaume, T. Gacoin and J. P. Boilot, J. Mater. Chem., 2006, 16, 529 RSC.
- F. Zhao, H. L. Sun, G. Su and S. Gao, Small, 2006, 2, 244 CrossRef CAS.
- M. J. Bierman, K. M. van Heuvelen, D. Schmeisser, T. C. Brunold and S. Jin, Adv. Mater., 2007, 19, 2677 CrossRef CAS.
- K. Kompe, H. Borchert, J. Storz, A. Lobo, S. Adam, T. Möller and M. Haase, Angew. Chem., Int. Ed., 2003, 42, 5513 CrossRef.
- T. X. T. Sayle, S. C. Parker and D. C. Sayle, Chem. Commun., 2004, 2438 RSC.
- S. W. Yang and L. Gao, J. Am. Chem. Soc., 2006, 128, 9330 CrossRef CAS.
- L. Yan, R. B. Yu, J. Chen and X. R. Xing, Cryst. Growth Des., 2008, 8, 1474 CrossRef CAS.
- J. Zhang, S. Ohara, M. Umetsu, T. Naka, Y. Hatakeyama and T. Adschiri, Adv. Mater., 2007, 19, 203 CrossRef CAS.
- Z. Q. Yang, K. B. Zhou, X. W. Liu, Q. Tian, D. Y. Lu and S. Yang, Nanotechnology, 2007, 18, 185606 CrossRef.
- Z. L. Wang and X. D. Feng, J. Phys. Chem. B, 2003, 107, 13563 CrossRef CAS.
- R. Si, Y. W. Zhang, L. P. You and C. H. Yan, Angew. Chem., Int. Ed., 2005, 44, 3256 CrossRef CAS.
- S. Kuchibhatla, A. S. Karakoti and S. Seal, Nanotechnology, 2007, 18, 075303 CrossRef.
- Y. N. Xia, P. D. Yang, Y. G. Sun, Y. Y. Wu, B. Mayers, B. Gates, Y. D. Yin, F. Kim and Y. Q. Yan, Adv. Mater., 2003, 15, 353 CrossRef CAS.
- X. Wang and Y. D. Li, Angew. Chem., Int. Ed., 2002, 41, 4790 CrossRef CAS.
- Y. W. Zhang, J. H. Liu, R. Si, Z. G. Yan and C. H. Yan, J. Phys. Chem. B, 2005, 109, 18324 CrossRef CAS.
- J. C. Wang, Q. Liu and Q. F. Liu, Opt. Mater., 2007, 29, 593 CrossRef CAS.
- Y. P. Fang, A. W. Xu, R. Q. Song, H. X. Zhang, L. P. You, J. C. Yu and H. Q. Liu, J. Am. Chem. Soc., 2003, 125, 16025 CrossRef CAS.
- Y. W. Zhang, Z. G. Yan, L. P. You, R. Si and C. H. Yan, Eur. J. Inorg. Chem., 2003, 4099 CrossRef CAS.
- Z. G. Yan, Y. W. Zhang, L. P. You, R. Si and C. H. Yan, J. Cryst. Growth, 2004, 262, 408 CrossRef CAS.
- R. X. Yan, X. M. Sun, X. Wang, Q. Peng and Y. D. Li, Chem.–Eur. J., 2005, 11, 2183 CrossRef CAS.
- H. X. Mai, Y. W. Zhang, L. D. Sun and C. H. Yan, J. Phys. Chem. C, 2007, 111, 13721 CrossRef CAS.
- Z. Y. Huo, C. Chen, D. Chu, H. H. Li and Y. D. Li, Chem.–Eur. J., 2007, 13, 7708 CrossRef CAS.
- H. Meyssamy, K. Riwotzki, A. Kornowski, S. Naused and M. Haase, Adv. Mater., 1999, 11, 840 CrossRef CAS.
- H. X. Mai, Y. W. Zhang, L. D. Sun and C. H. Yan, Chem. Mater., 2007, 19, 4514 CrossRef CAS.
- T. Fukuyo and H. Imai, J. Cryst. Growth, 2002, 241, 193 CrossRef CAS.
- H. W. Song, L. X. Yu, S. Z. Lu, T. Wang, Z. X. Liu and L. M. Yang, Appl. Phys. Lett., 2004, 85, 470 CrossRef CAS.
- K. Riwotzki and M. Haase, J. Phys. Chem. B, 1998, 102, 10129 CrossRef CAS.
- A. Huignard, T. Gacoin and J. P. Boilot, Chem. Mater., 2000, 12, 1090 CrossRef CAS.
- K. Riwotzki and M. Haase, J. Phys. Chem. B, 2001, 105, 12709 CrossRef CAS.
- C. J. Jia, L. D. Sun, F. Luo, X. C. Jiang, L. H. Wei and C. H. Yan, Appl. Phys. Lett., 2004, 84, 5305 CrossRef CAS.
- C. J. Jia, L. D. Sun, L. P. You, X. C. Jiang, F. Luo, Y. C. Pang and C. H. Yan, J. Phys. Chem. B, 2005, 109, 3284 CrossRef CAS.
- F. Luo, C. J. Jia, W. Song, L. P. You and C. H. Yan, Cryst. Growth Des., 2005, 5, 137 CrossRef CAS.
- J. W. Stouwdam, M. Raudsepp and F. C. J. M. van Veggel, Langmuir, 2005, 21, 7003 CrossRef CAS.
- J. F. Liu and Y. D. Li, Adv. Mater., 2007, 19, 1118 CrossRef CAS.
- H. Deng, S. H. Yang, S. Xiao, H. M. Gong and Q. Q. Wang, J. Am. Chem. Soc., 2008, 130, 2032 CrossRef CAS.
- S. Heer, K. Kompe, H. U. Gudel and M. Haase, Adv. Mater., 2004, 16, 2102 CrossRef CAS.
- Y. Cheng, Y. S. Wang, Y. H. Zheng and Y. Qin, J. Phys. Chem. B, 2005, 109, 11548 CrossRef CAS.
- Y. C. Cao, J. Am. Chem. Soc., 2004, 126, 7456 CrossRef CAS.
- R. Si, Y. W. Zhang, H. P. Zhou, L. D. Sun and C. H. Yan, Chem. Mater., 2007, 19, 18 CrossRef CAS.
- Y. P. Du, Y. W. Zhang, L. D. Sun and C. H. Yan, J. Phys. Chem. C, 2008, 112, 405 CrossRef CAS.
- Y. P. Du, Y. W. Zhang, Z. G. Yan, L. D. Sun, S. Gao and C. H. Yan, Chem. Asian J., 2007, 2, 965 CrossRef CAS.
- X. Sun, Y. W. Zhang, R. Si and C. H. Yan, Small, 2005, 1, 1081 CrossRef CAS.
- J. W. Stouwdam and F. C. J. M. van Veggel, Nano Lett., 2002, 2, 733 CrossRef CAS.
- N. Stubicar, Cryst. Growth Des., 2005, 5, 113 CrossRef CAS.
- Y. W. Zhang, X. Sun, R. Si, L. P. You and C. H. Yan, J. Am. Chem. Soc., 2005, 127, 3260 CrossRef CAS.
- X. Sun, Y. W. Zhang, Y. P. Du, Z. G. Yan, R. Si, L. P. You and C. H. Yan, Chem.–Eur. J., 2007, 13, 2320 CrossRef CAS.
- F. Zhao, M. Yuan, W. Zhang and S. Gao, J. Am. Chem. Soc., 2006, 128, 11758 CrossRef CAS.
- F. Zhao, H. L. Sun, S. Gao and G. Su, J. Mater. Chem., 2005, 15, 4209 RSC.
- J. F. Liu and Y. D. Li, Adv. Mater., 2006, 19, 1118.
- Z. H. Li, J. H. Zeng and Y. D. Li, Small, 2007, 3, 438 CrossRef CAS.
- M. Yada, C. Taniguchi, T. Torikai, T. Watari, S. Furuta and H. Katsuki, Adv. Mater., 2004, 16, 1448 CrossRef CAS.
- F. Maurel, M. J. Hytch, B. Knosp and M. Backhaus-Ricoult, Eur. Phys. J.: Appl. Phys., 2000, 9, 205 CrossRef CAS.
- H. P. Yang, D. S. Zhang, L. Y. Shi and J. H. Fang, Acta Mater., 2008, 56, 955 CrossRef CAS.
- X. Wang and Y. D. Li, Chem.–Eur. J., 2003, 9, 5627 CrossRef CAS.
- X. Wang, X. M. Sun, D. P. Yu, B. S. Zou and Y. D. Li, Adv. Mater., 2003, 15, 1442 CrossRef CAS.
- M. Yada, M. Mihara, S. Mouri, M. Kuroki and T. Kijima, Adv. Mater., 2002, 14, 309 CrossRef CAS.
- C. F. Wu, W. P. Qin, G. S. Qin, D. Zhao, J. S. Zhang, S. H. Huang, S. Z. Lu, H. Q. Liu and H. Y. Lin, Appl. Phys. Lett., 2003, 82, 520 CrossRef CAS.
- C. C. Tang, Y. Bando, D. Golberg and R. Z. Ma, Angew. Chem., Int. Ed., 2005, 44, 576 CrossRef CAS.
- C. X. Guo, M. H. Cao and C. W. Hu, J. Nanosci. Nanotechnol., 2005, 5, 184 CrossRef CAS.
- W. D. Shi, J. B. Yu, H. H. Wang, J. H. Yang and H. J. Zhang, J. Nanosci. Nanotechnol., 2006, 6, 2515 CrossRef CAS.
- W. J. Li, X. Wang and Y. D. Li, Chem. Commun., 2004, 164 RSC.
- A. W. Xu, Y. P. Fang, L. P. You and H. Q. Liu, J. Am. Chem. Soc., 2003, 125, 1494 CrossRef CAS.
- G. Z. Chen, C. X. Xu, X. Y. Song, W. Zhao, Y. Ding and S. X. Sun, Inorg. Chem., 2008, 47, 723 CrossRef CAS.
- Y. B. Mao, J. Y. Huang, R. Ostroumov, K. L. Wang and J. P. Chang, J. Phys. Chem. C, 2008, 112, 2278 CrossRef CAS.
- P. Martin, S. C. Parker, D. C. Sayle and G. W. Watson, Nano Lett., 2007, 7, 543 CrossRef.
- F. Zhang, Y. Wan, T. Yu, F. Q. Zhang, Y. F. Shi, S. H. Xie, Y. G. Li, L. Xu, B. Tu and D. Y. Zhao, Angew. Chem., Int. Ed., 2007, 46, 7976 CrossRef CAS.
- L. F. Liang, H. F. Xu, Q. Su, H. Konishi, Y. B. Jiang, M. M. Wu, Y. F. Wang and D. Y. Xia, Inorg. Chem., 2004, 43, 1594 CrossRef CAS.
- C. J. Jia, L. D. Sun, Z. G. Yan, Y. C. Pang, L. P. You and C. H. Yan, J. Phys. Chem. C, 2007, 111, 13022 CrossRef CAS.
- X. Wang and Y. D. Li, Angew. Chem., Int. Ed., 2003, 42, 3497–3500 CrossRef CAS.
- H. C. Lu, G. S. Yi, S. Y. Zhao, D. P. Chen, L. H. Guo and J. Cheng, J. Mater. Chem., 2004, 14, 1336 RSC.
- S. Heer, O. Lehmann, M. Haase and H. U. Gudel, Angew. Chem., Int. Ed., 2003, 42, 3179 CrossRef CAS.
- T. Hirai and T. Orikoshi, J. Colloid Interface Sci., 2004, 269, 103 CrossRef CAS.
- J. H. Zeng, J. Su, Z. H. Li, R. X. Yan and Y. D. Li, Adv. Mater., 2005, 17, 2119 CrossRef CAS.
- J. C. Boyer, F. Vetrone, L. A. Cuccia and J. A. Capobianco, J. Am. Chem. Soc., 2006, 128, 7444 CrossRef CAS.
- H. X. Mai, Y. W. Zhang, R. Si, Z. G. Yan, L. D. Sun, L. P. You and C. H. Yan, J. Am. Chem. Soc., 2006, 128, 6426 CrossRef CAS.
- C. X. Li, Z. W. Quan, J. Yang, P. P. Yang and J. Lin, Inorg. Chem., 2007, 46, 6329 CrossRef CAS.
- H. X. Mai, Y. W. Zhang, L. D. Sun and C. H. Yan, J. Phys. Chem. C, 2007, 111, 13730 CrossRef CAS.
- A. Aebischer, S. Heer, D. Biner, K. Kramer, M. Haase and H. U. Gudel, Chem. Phys. Lett., 2005, 407, 124 CrossRef CAS.
- Z. G. Wei, L. D. Sun, C. S. Liao, X. C. Jiang and C. H. Yan, J. Mater. Chem., 2002, 12, 3665 RSC.
- X. C. Jiang, L. D. Sun, W. Feng and C. H. Yan, Cryst. Growth Des., 2004, 4, 517 CrossRef CAS.
- X. C. Jiang, L. D. Sun and C. H. Yan, J. Phys. Chem. B, 2004, 108, 3387 CrossRef CAS.
- Y. Z. Huang, L. Chen and L. M. Wu, Cryst. Growth Des., 2008, 8, 739 CrossRef CAS.
- H. X. Mai, L. D. Sun, Y. W. Zhang, R. Si, W. Feng, H. P. Zhang, H. C. Liu and C. H. Yan, J. Phys. Chem. B, 2005, 109, 24380 CrossRef CAS.
- H. Liu, C. G. Hu and Z. L. Wang, Nano Lett., 2006, 6, 1535 CrossRef CAS.
- C. G. Hu, H. Liu, W. T. Dong, Y. Y. Zhang, G. Bao, C. S. Lao and Z. L. Wang, Adv. Mater., 2007, 19, 470 CrossRef CAS.
- Z. W. Zhang, C. G. Hu, Y. F. Xiong, R. S. Yang and Z. L. Wang, Nanotechnology, 2007, 18, 465504 CrossRef.
- G. H. Pan, H. W. Song, X. Bai, Z. X. Liu, H. Q. Yu, W. H. Di, S. W. Li, L. B. Fan, X. G. Ren and S. Z. Lu, Chem. Mater., 2006, 18, 4526 CrossRef CAS.
- Y. S. Lin, Y. Hung, H. Y. Lin, Y. H. Tseng, Y. F. Chen and C. Y. Mou, Adv. Mater., 2007, 19, 577 CrossRef CAS.
- R. Si and M. Flytzani-Stephanopoulos, Angew. Chem., Int. Ed., 2008, 47, 2884 CrossRef CAS.
- L. Liao, H. X. Mai, Q. Yuan, H. B. Lu, J. C. Li, C. Liu, C. H. Yan, Z. X. Shen and T. Yu, J. Phys. Chem. C, 2008, 112, 9061 CrossRef CAS.
- H. P. Zhou, Y. W. Zhang, H. X. Mai, X. Sun, Q. Liu, W. G. Song and C. H. Yan, Chem.–Eur. J., 2008, 14, 3380 CrossRef CAS.
- Q. Yuan, Q. Liu, W. G. Song, W. Feng, W. L. Pu, L. D. Sun, Y. W. Zhang and C. H. Yan, J. Am. Chem. Soc., 2007, 129, 6698 CrossRef CAS.
- E. V. Shevchenko, D. V. Talapin, N. A. Kotov, S. O'Brien and C. B. Murray, Nature, 2006, 439, 55 CrossRef.
- N. Y. Fogel, E. I. Buchstab, Y. V. Bomze, O. I. Yuzephovich, A. Y. Sipatov, E. A. Pashitskii, A. Danilov, V. Langer, R. I. Shekhter and M. Jonson, Phys. Rev. B, 2002, 66, 174513 CrossRef.
- F. X. Redl, K. S. Cho, C. B. Murray and S. O'Brien, Nature, 2003, 423, 968 CrossRef CAS.
- P. D. Yang, Nature, 2003, 425, 243 CrossRef CAS.
- S. H. Sun, S. Anders, H. F. Hamann, J. U. Thiele, J. E. E. Baglin, T. Thomson, E. E. Fullerton, C. B. Murray and B. D. Terris, J. Am. Chem. Soc., 2002, 124, 2884 CrossRef CAS.
- J. Chun, J. A. Fagan, E. K. Hobbie and B. J. Bauer, Anal. Chem., 2008, 80, 2514 CrossRef CAS.
- S. F. Sweeney, G. H. Woehrle and J. E. Hutchison, J. Am. Chem. Soc., 2006, 128, 3190 CrossRef CAS.
- A. M. Gole, C. Sathivel, A. Lachke and M. Sastry, J. Chromatogr., A, 1999, 848, 485 CrossRef CAS.
- P. K. Rai, A. N. G. Parra-Vasquez, H. Peng, R. H. Hauge and M. Pasquali, J. Phys. Chem. C, 2007, 111, 17966 CrossRef CAS.
- E. V. Shevchenko, D. V. Talapin, C. B. Murray and S. O'Brien, J. Am. Chem. Soc., 2006, 128, 3620 CrossRef CAS.
- R. Si, Y. W. Zhang, L. P. You and C. H. Yan, J. Phys. Chem. B, 2006, 110, 5994 CrossRef CAS.
- S. J. Marrink, A. H. de Vries and A. E. Mark, J. Phys. Chem. B, 2004, 108, 750 CrossRef CAS.
- E. Lindahl, B. Hess and D. van der Spoel, J. Mol. Model., 2001, 7, 306 CAS.
- J. Paek, C. H. Lee, J. Choi, S. Y. Choi, A. Kim, J. W. Lee and K. Lee, Cryst. Growth Des., 2007, 7, 1378 CrossRef CAS.
- C. Daiguebonne, N. Kerbellec, O. Guillou, J. C. Bunzli, F. Gumy, L. Catala, T. Mallah, N. Audebrand, Y. Gerault, K. Bernot and G. Calvez, Inorg. Chem., 2008, 47, 3700 CrossRef CAS.
- N. Kerbellec, L. Catala, C. Daiguebonne, A. Gloter, O. Stephan, J. C. Bunzli, O. Guillou and T. Mallah, New J. Chem., 2008, 32, 584 RSC.
- A. S. Barnard and L. A. Curtiss, Nano Lett., 2005, 5, 1261 CrossRef CAS.
- J. Shen, L. D. Sun and C. H. Yan, Dalton Trans., 2008 10.1039/b805306e , in press.
| This journal is © The Royal Society of Chemistry 2008 |
