Multilayer microcapsules with tailored structures for bio-related applications†
Weijun
Tong
and
Changyou
Gao
*
Key Laboratory of Macromolecular Synthesis and Functionalization, Ministry of Education, Department of Polymer Science and Engineering, Zhejiang University, Hangzhou 310027, China. E-mail: cygao@mail.hz.zj.cn; Fax: +86-571-87951108; Tel: +86-571-87951108
First published on 2nd July 2008
Abstract
The technique for fabrication of hollow multilayer microcapsules is initially established by layer-by-layer (LbL) assembly of oppositely charged polyelectrolytes onto sacrificial colloidal particles followed by core removal. It has the advantages of precise control over the size, shape, composition, wall thickness and functions of the obtained hollow microcapsules. More recently, the technique has been developed fast from the fabrication and basic property investigation to functionalization and applications, in particular in bio-related fields such as controlled drug delivery, biosensors and bioreactors. In this article, we shall review recent advances in property manipulation of electrostatic and hydrogen bonded microcapsules by chemical crosslinking, and microcapsules assembled by other driving forces such as covalent interaction, base pair interaction, host–guest interaction and van der Waals interaction. Then the possible applications of LbL microcapsules in bio-related fields are summarized with particular emphasis on very recent achievements in terms of loading and release, surface modification for stealth function or targeting and use as biosensors and bioreactors.
 Weijun Tong | Weijun Tong is currently a lecturer of material science at Zhejiang University. He obtained his PhD in material science in 2007 under the supervision of Prof. Changyou Gao at Zhejiang University, China and Prof. Helmuth Möhwald at the Max-Planck Institute of Colloids and Interfaces, Germany. His main scientific interests are supramolecular assembly and microcapsules and their applications in the biomedical field. |
 Changyou Gao | Changyou Gao is currently a professor of material science at Zhejiang University. He got his PhD in polymer chemistry and physics in 1996 under the supervision of Prof. Jiacong Shen at Jilin University, China. His research interests include self-assembled microcapsules, biomaterials and nanomaterials for biomedical applications. |
1. Introduction
Hollow capsules are of great interest due to their potential applications and fundamental importance as new colloidal structures in areas such as medicine, catalysis, cosmetics as well as biotechnology.1 Recently, a novel type of hollow microcapsule has been fabricated by means of layer-by-layer (LbL) assembly of oppositely charged polyelectrolytes2,3 on colloidal templates, followed by core removal (Fig. 1).4–6 Using this technique, capsules with well-controlled size and shape, finely tuned wall thickness and variable wall compositions have been produced.7 Moreover, microcapsules with customized physicochemical properties can be obtained by incorporation of one or more functional species such as biomacromolecules,8 lipids,9 photoactive dyes,10nanoparticles,11 as well as multivalent ions12 onto the capsule walls or into the capsule interiors. The LbL microcapsules with integrated multifunctionalities have a high capacity for loading a wide range of substances and a sensitive response to diverse stimuli, thus they are highly attractive for bio-related applications.13–15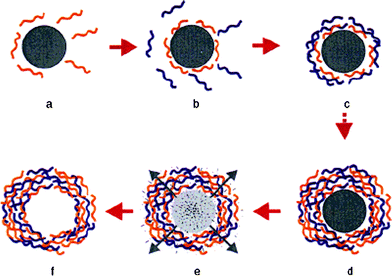 | ||
| Fig. 1 Schematic illustration of the polyelectrolyte deposition process and of subsequent core decomposition. Reprinted with permission from ref. 5. Copyright 1998, Wiley-VCH. | ||
Up to now, several excellent articles have reviewed the progress of LbL microcapsules with respect to fabrication and physicochemical properties,7,16 permeability,17–19 loading and release,18–21 serving as microreactors,19,22 mechanical properties23–25 and biofunctionality.26,27 In particular, the inorganic reactions inside the polyelectrolyte microcapsules to synthesize magnetic, fluorescent and mineral nanoparticles have clear relevance to the bio-related applications, which has been covered by a recent comprehensive review.22 Readers can get the respective overviews there and those works shall not be repeated here. In this article, we focus on the very recent progress of the LbL microcapsules with respect to manipulation of their properties by chemical crosslinking, fabrication of the microcapsules based on new driving forces such as covalent interaction, base pair interaction, host–guest interaction and van der Waals interaction, and their bio-related functions and applications for loading and release, anti-endocytosis or targeting, biosensors and bioreactors.
2 Advances in fabrication of multilayer microcapsules by LbL techniques
2.1 Crosslinking to manipulate the properties of LbL microcapsules based on electrostatic interaction and hydrogen bonding
Although electrostatic interaction is generally strong enough to hold the integrity of LbL microcapsules, it is still susceptible to high ionic strength, extreme pH and strong polar organic solvents, and the properties of microcapsules may thus become adverse, consequently influencing the applications. Crosslinking of the capsule walls is an effective way to manipulate their properties such as permeability, mechanical properties and stability. Several crosslinking methods have been developed, most of which were initially used for multilayer films and subsequently for LbL microcapsules. For example, Bruening and co-workers used a simple heat induced amide formation method to crosslink the poly(allylamine hydrochloride) (PAH)/poly(acrylic acid) (PAA) multilayers.28 The crosslinked, nylon-like films are stable over a wide pH range and highly impermeable. Zhang and co-workers constructed diazoresins (DARs) and poly(sodium styrene sulfonate) (PSS) multilayers, which can form a covalently crosslinked structure by UV irradiation.29 The films have no detectable damage when sonicated in H2O–dimethyl formamide (DMF)–ZnCl2 ternary solvent for 0.5 h, while the uncrosslinked films lose 30% of their material when incubated in the same solvent for 5 min. These methods are also applicable to microcapsules and can induce stable structures. For example, the PAH/PAA microcapsules crosslinked by amide formation at high temperature are rather stable in a wide pH range.30 The DAR/PSS microcapsules crosslinked by UV radiation have a higher mechanical stability measured by a method of osmotic-induced invagination,31,32 and are more stable in various chemical environments than their uncrosslinked counterparts.33 The crosslinked DAR/PSS capsules can also effectively reduce the cut-off molecular weight of the capsule walls, thus enzymes can be encapsulated after the crosslinking.34 Moreover, carbodiimide chemistry has also been used to crosslink weak polyelectrolyte microcapsules containing carboxylic and amine groups, yielding stable structures.35,36The above mentioned techniques are exclusively based on a reaction between the functional groups of the two components in the multilayers. We recently demonstrated that the multilayer microcapsules assembled from PAH and PSS could be considerably stabilized by crosslinking only the PAH component with glutaraldehyde (GA).37 After crosslinking by 2% GA for 2 h, an apparently thicker capsule wall was obtained with higher folds. No alteration of the macroscopic topology of the capsules was observed after incubation in 0.1 M NaOH for 24 h. The crosslinking can significantly improve the mechanical strength of the capsules to resist osmotic pressure induced invagination. Consequently, both the critical pressure and the elasticity modulus (680 MPa) of the capsule walls are doubled compared with that of the control. The crosslinking can also greatly lower the permeability of the capsule walls. Quantitative analysis revealed that the permeation coefficient for dextran (Mw ≈ 250 kDa) was reduced by a factor of 3 after crosslinking. We further applied this method to the poly(ethylenimine) (PEI) and PAA weak polyelectrolyte microcapsules.38 The crosslinked microcapsules can maintain their macroscopic topology at extreme low or high pH, while reorganizing their microstructures to enable selective permeation or rejection of macromolecules at lower (pH<4) and higher pH (pH>6), respectively. Using this property, dextran with a molecular weight of 2000 kDa was successfully encapsulated. Thus, it is possible to produce capsules that are at the same time pH-responsive as well as stable over a large pH range.
Hydrogen bonding is the first non-electrostatic interaction used in theLbL assembly. More information about this topic can be found from a recent review paper and the references therein.39 It is well known that the hydrogen bonded multilayers will disassemble at physiological conditions.40,41 When they are used for biomedical applications, there is a need to stabilize the film too. The most commonly used method for crosslinking of the hydrogen bonded multilayers and capsules to date is the carbodiimide chemistry, which induces the formation of amide linkages.42,43 For example, stability of the hydrogen bonded multilayer microcapsules is improved by the carbodiimide chemistry using ethylenediamine as a foreign crosslinking reagent for the poly(methacrylic acid) component.44 The uncrosslinked component can be selectively released at elevated pH, yielding single component and hydrogel like microcapsules.45,46 These capsules exhibit reversible pH-responsive swelling and shrinking, which can be used for loading and release of macromolecules. Revisable stabilization of hydrogen bonded LbL capsulesviadisulfide bonds has also been reported.47,48 The crosslinked films are stable in neutral or alkaline solutions, but disassemble rapidly under reductive conditions.
2.2 LbL capsules based on other driving forces
It is known that different driving forces bring substantial differences to the microcapsules in terms of their chemical and physical structures, stability and stimuli-response, and inevitably their functionality and applicability. The driving forces for LbL assembly of microcapsules mainly began with electrostatic interaction,4–6 which limits the building blocks to a narrow range of charged species. Then hydrogen bonding has been applied to synthesis of core–shell particles and hollow capsules as discussed above. Recently, the multilayer hollow microcapsules based on covalent bonding, base pair interactions, van de Waals interactions and guest–host interactions have been fabricated, which show unique properties.The first example of covalent LbL assembled microcapsules was demonstrated by Xu and co-workers.49N-Methyl-2-nitro-diphenylamine-4-diazoresin (NDR) and m-methylphenolformaldehyde resin (MPR) dissolved in methanol were alternately adsorbed on polystyrene (PS) microparticles through direct covalent reaction. Hollow capsules were obtained after removal of the PS particles. These capsules are stable enough to withstand the long etching time of strong polar organic solvents. The use of LbL strategy from aqueous systems to nonaqueous systems greatly widens the range of materials used for LbL assembly. We recently fabricated a new structure of microcapsules with high modulus and high stability through covalent LbL assembly (Fig. 2).50Aminosilanized SiO2 microparticles were used as templates. Poly(glycidyl methylacrylate) (PGMA) and PAH were alternately immobilized onto the particle surfaces through a coupling reaction between the epoxides and the amines. Thus, a highly crosslinked structure was produced in this process. When the desired layer number was reached, the particles were removed by HF solution, resulting in hollow microcapsules. The microcapsules are stable in extreme pHs and at elevated temperature. Using the method of osmotic-induced invagination,31,32 the elastic modulus of the microcapsule walls without any treatments was found to be as high as 910 MPa. The acid- and base-treatment cannot decrease the modulus of the microcapsule walls.
 | ||
| Fig. 2 Schematic illustration of the process of direct covalent LbL assembly on a silica particle, and fabrication of a hollow capsule by etching out the template core. The blue lines represent PGMA, the red lines represent PAH, and the green dots represent the covalent linkage between layers. Reprinted with permission from ref. 50. Copyright 2007, Wiley-VCH. | ||
Making use of the features of the reaction between amine and aldehyde, i.e. fast and efficient in aqueous solution at room temperature, we recently developed a method to fabricate single polyelectrolyte component multilayers and microcapsules through direct covalent assembly of PAH with a low molecular weight dialdehyde linker -GA.51 The structure as well as the cut-off molecular weight of the capsule walls can be tuned by the molecular weight of the polymer blocks.52 Because GA can readily react not only with amino groups but also with hydroxyl groups under very mild conditions, this method can be extended to other macromolecules, especially biomacromolecules such as polysaccharides, polypeptides, and proteins as well. Stimuli-responsive covalent LbL assembled microcapsules have been recently reported by Caruso and co-workers.53 They used the click chemistry approach to form multilayer films54 (Fig. 3) on particles and obtained hollow microcapsules after core removal. PAA grafted with a minor amount of either alkyne (PAA-Alk) or azide (PAA-Az) were alternately assembled on silica particles through the click reaction between the Alk and Az. Due to the nature of the weak polyelectrolyte PAA in the shells, the capsules can reversibly swell and shrink by up to 70% when subjected to acid–base pH cycling. These two methods can fabricate single polymer component LbL capsules, thus are helpful in correlating the structures and their properties.
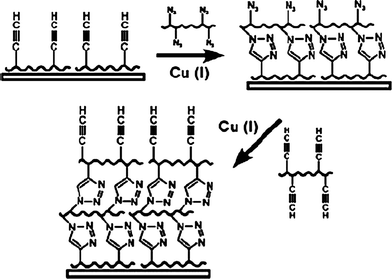 | ||
| Fig. 3 LbL assembly of multilayer films using click chemistry. Reprinted with permission from ref. 54. Copyright 2006, American Chemical Society. | ||
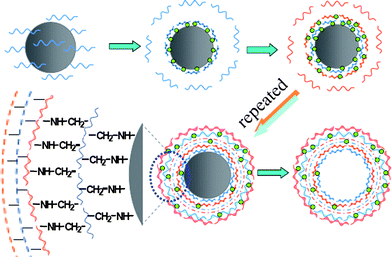
Host–guest interaction is another type of driving force frequently employed in supramolecular chemistry. The complex usually forms between two determined molecules, typically exemplified by cyclodextrin (CD) and its guests.55–57 A few attempts have been made to build LbL multilayer films using this interaction.58–60 It is known that the host–guest interaction is readily mediated by the host and guest molecules with respect to their matching degree and concentration. If charge interaction is further introduced, multi-responsive microcapsules can thus be expected. According to this design, multilayer microcapsules were fabricated by using the interaction between β-cyclodextrin (β-CD) and ferrocene grafted to a weak polyelectrolyte PAH, which can further introduce charge interaction into the capsule walls (Fig. 4).61 The microcapsules that consist of PAH-g-β-CD and PAH-g-ferrocene indeed show multi-responsiveness to environmental stimuli. For example, they swell and shrink at low and high pH, respectively. Incubation in a salt or β-CD solution can also mediate their swelling and shrinking behavior. With these smart features, the microcapsules can serve as reservoirs for drugs, DNAs, enzymes and so on.
 | ||
| Fig. 4 LbL assembly of the same polyelectrolyte on carbonate particles to obtain hollow microcapsules using host–guest interactions. The chemical structure of PAH-g-β-CD, PAH-g-ferrocene, and β-CD/ferrocene inclusive are shown in the second row. | ||
Assembly of DNA multilayer films both on planar substrates and colloidal particles from engineered sequences through specific base pair interaction has been demonstrated recently by Caruso’s group.62–64 It was found that the composition and sequences of the oligonucleotides play an important role in the structure and assembly of the DNA multilayer films. After removal of the colloidal particles, hollow DNA capsules were obtained and their shrinkage could be controlled by the composition of the DNA sequences used for the multilayers’ assembly.64 The shrinkage of the capsules can be used for encapsulation of desirable substances.65
Akashi et al. recently reported the preparation of stable, ultrathin films of a double stranded poly(methyl methacrylate) (PMMA) stereocomplex by the alternate LbL assembly of it- and st-PMMA on a solid substrate (it denoted isotactic, st denotes syndiotactic).66 Later on, the same group applied the it-/st-PMMA stereocomplex films onto silica particles and obtained hollow capsules of nonionic multilayers based on van der Waals interactions after core removal.67 However, the specific properties correlated with the nature of the van der Waals interactions need further exploration.
3 Bio-related applications of multilayer microcapsules
3.1 Delivery carriers
For most of the potential applications of LbL microcapsules such as drug delivery, catalysis, cosmetics, biotechnology and nutrition, the controlled loading and release of a variety of substances is basically required. As aforementioned, since several review papers17,20,21 have focused on this subject, we introduce here only the very recent progress of the related works.The spontaneous deposition is based on a mechanism of high affinity of the preloaded substances with the substances of interest. This is initially driven by an interesting phenomenon that positively charged molecules such as dextran labeled with tetramethylrhodamine isothiocyanate (TRITC-dextran) can largely deposit into the aged “hollow” microcapsules templated on melamine formaldehyde (MF) particles.85 By comparison of the fluorescence intensity from the capsule interiors to the capsule surroundings, it is obvious that a considerably high concentration of dextran in the capsule interiors has been achieved (the so-called spontaneous deposition) (Fig. 5a). Many other water-soluble substances with positive charges such as polyelectrolytes,84proteins,84 enzymes88 and low molecular weight dyes and anti-cancer drugs (Fig. 5b, c)85 can be spontaneously deposited with a large of quantity (the interior concentration is tens to more than hundred times higher than that of the bulk). Moreover, the deposition still occurs even if the molecules have very weak positive charge such as the TRITC-dextran (Fig. 5a), which is generally regarded as a neutral molecule but actually gains slight positive charges from a few pendent TRITC groups. The driving force for this phenomenon is attributed to the existence of a negatively charged complex (PSS/MF) within the capsule interior, which is formed by the dissociated PSS from the very initial layer and the positively charged MF degradation product (Fig. 6). It is worth noting that black rings emerged in between the capsule walls and the deposits (Fig. 5a, inset). This phenomenon is typical for spontaneous deposition, which might reflect the distribution of the negatively charged complex. The charge repulsion between the complex and the inner capsule wall (negatively charged PSS as the first layer) may lead to the floating of the complex in the capsule interior rather than attachment onto the capsule wall.
 | ||
| Fig. 5 (a) Fluorescence intensity averaged from inside the circles as a function of incubation time. TRITC-dextran (Mw ≈ 65 kDa) and preformed MF-(PSS/PAH)5 capsules were used. (b) TEM images of Daunorubicin (DNR) deposited MF-(PSS/PAH)5 capsules. (c) DNR and rhodamine B (RdB) concentrations in the capsule interior as a function of temperature. MF-(PSS/PAH)4(PSS/PDADMAC)5 capsules were used for DNR with a feeding concentration of 30 mg ml−1, and MF-(PSS/PAH)5 capsules for RdB, 80 mg ml−1. The numbers in the figure represent the concentration ratios of the capsule interior and the bulk. PDADMAC = polydiallyldimethyl ammonium chloride. Reprinted with permission from ref. 85. Copyright 2005, Wiley-VCH. | ||
 | ||
| Fig. 6 Schematic illustration to show the microstructure of the polyelectrolyte capsule containing a trace amount of negatively charged MF/PSS complex, by which positively charged DNR and RdB are sucked into the capsule interior to form a higher concentration than that of the bulk (the so-called spontaneous deposition). Reprinted with permission from ref. 85. Copyright 2005, Wiley-VCH. | ||
By pre-filling the microcapsules with charged polyelectrolytes, loading of oppositely charged substances with a higher concentration in the capsule interiors can be similarly achieved. For example, due to the electrostatic interaction of positively charged doxorubicin hydrochloride (DOX) and negatively charged dextran sulfate, the loading amount of DOX increases about 3-fold when the capsules are filled with low molecular weight dextran sulfate relative to that of the hollow ones.86
The coprecipitation method was first reported by Sukhorukov and co-workers.87 A Na2CO3 solution was added to an equal volume of a CaCl2 solution with the same concentration containing the macromolecules such as proteins and enzymes, which were then coprecipitated during the CaCO3 microparticle growth. Core removal after LbL assembly of the multilayers leads to release of the macromolecules into the polyelectrolyte microcapsule interiors. The mild fabricating conditions can largely preserve the enzymatic activity of encapsulated α-chymotrypsin up to 85% by this method. The concentration of the loaded substances can be easily controlled by their initial concentrations in the mixed reaction solution, and much larger amounts of the macromolecules can be achieved. Moreover, a wide range of substances such as polyelectrolytes,89,90 enzymes,91 DNAs,91polysaccharides92 and micelles93 have been encapsulated by this method
More recently, we found that the capsules filled with PSS obtained by the coprecipitation method can effectively sieve charged molecules (Fig. 7).89,90 While the microcapsules reject the permeation of negatively charged species, they can attract the deposition of positively charged molecules. The selectivity is very sensitive. For example, the capsules completely exclude dextran labeled with fluorescein isothiocyanate (FITC-dextran), but are permeable to TRITC-dextran having a similar molecular weight (from 4 kD to 70 kD), although there are only few charged dyes in a dextran chain.
 | ||
| Fig. 7 (a) Schematic illustration of the topology of PSS encapsulated microcapsules. (b) Scanning force microscopy (SFM) image of an intact capsule with free PSS trapped inside. Confocal laser scanning microscopy (CLSM) images of the PSS filled microcapsules incubated in solutions of (a) fluorescein for 2 h and (b) rhodamine 6 G for a few seconds. Reprinted with permission from ref. 89. Copyright 2005, American Chemical Society. | ||
The most straightforward way to realize burst release is to destroy the capsule wall by external or internal stimuli. For example, some metal nanoparticles and dyes incorporated into the multilayer capsule walls can absorb near-infrared lasers to induce a local temperature increase and destroy the walls, leading to burst release.94–98Ultrasonication is another way which can remotely destroy the capsule walls rapidly (in 5 s).99,100 The presence of magnetite nanoparticles in the capsule walls can enable magnetically controlled delivery to the desired site and significantly improve the efficiency of the ultrasonically stimulated release of their content. It is notable that the encapsulated substances can be released at an ultrasonic power comparable to those applied in medicine, demonstrating the practical applicability of this novel method.
The decomposition of the capsule walls also can be triggered by specific chemical stimuli. For example, the capsules based on hydrogen bonding and crosslinked by disulfide bonds are stable at normal physiological conditions. The disulfide bond can be cleaved in a reducing environment, inducing the immediate deconstruction of the hydrogen bonded multilayer capsules and the release of loaded susbtances.47,48 De Smedt and co-workers synthesized a polyelectrolyte containing phenylboronic acid as a glucose-sensitive moiety, with which multilayer microcapsules having glucose-sensitivity were assembled.101 In a solution containing 2.5 mg ml−1 or 5 mg ml−1glucose the capsules were dissolved within 5 min. Therefore, this kind of capsule is very promising for delivery of glucose related drugs such as insulin. The same group also reported the “self-exploding” microcapsule, which is composed of a biodegradable dextran gel core surrounded by a polyelectrolyte membrane.102 The gel can be hydrolyzed to yield products which are not permeable through the capsule wall. Consequently, when the osmotic pressure inside the capsule is high enough, the polyelectrolyte membrane is ruptured to release the loaded substances. Later on, the same group reported on degradable polyelectrolyte capsules containing one or two polyelectrolytes which can be degraded either enzymatically or through hydrolysis.92 After these capsules are internalized by cells, they will be subsequently degraded and release the encapsulated substances into the cells.
Sustained release of loaded materials can be accomplished by gradual decomposition of capsule walls or slow diffusion through capsule walls. For instance, biodegradable hollow capsules loaded with proteins have been prepared viaLbL assembly of chitosan and dextran sulfate on protein-entrapped mesoporous silica particles and the subsequent core removal.103 In the presence of chitosanase, the chitosan component can be gradually degraded with time prolongation, and the capsules begin to deform and are finally destroyed. Thus the encapsulated proteins are released in a sustained manner and the release behavior can be manipulated by the chemical compositions of the capsule surface. The enzymatic deconstruction of LbL capsules also can be realized through incorporation of enzymes into their interiors. For example, Kreft and co-workers prepared self-disintegrating microcapsules by encapsulating a highly active mix of proteases into polypeptide LbL microcapsules (Fig. 8).91 Pronase was coprecipitated into CaCO3 microparticles that were subsequently coated with poly(L-arginine)/poly(L-aspartic acid) multilayers. Then the enzyme was released into the capsule interiors and started to digest the capsule walls after core removal. The biggest advantage of this method is that by varying the amount of encapsulated pronase, lifetimes of such self-disintegrating capsules can be successfully adjusted from seconds and hours to days, thus the sustained release of the co-encapsulated DNA can be adjusted.
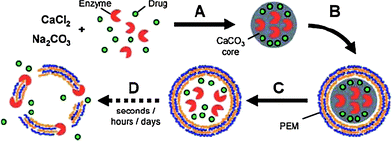 | ||
| Fig. 8 Principle of time-delayed drug release from enzyme-degradable polyelectrolyte capsules. (A) Co-immobilization of a polyelectrolyte degrading enzyme and drug molecules in CaCO3 microparticles. (B) LbL coating with specific, enzyme-degradable polyelectrolytes. (C) Core-dissolution leads to release of enzyme and drug into the inner void of the capsule. (D) Enzyme digests capsule-shell and releases drug. Reprinted with permission from ref. 91. Copyright 2007, Wiley-VCH. | ||
The spontaneously deposited low molecular weight drugs in the LbL microcapsules templated on MF colloidal particles can be sustainably released by diffusion through the capsule walls.85,104 The amount of loaded drugs is proportional to the drug feeding concentration, temperature and salt concentration, demonstrating tailorable deposition behavior that is crucial for the drug delivery vehicle. The deposited drugs could be released again in a sustained manner and their behaviors can be manipulated by changing the interaction between the drugs and the PSS/MF complex existing in the hollow capsules by ionic strength, pH and temperature. The presence of anti-cancer drug loaded capsules was seen to steadily decrease the cytoviability of HL-60 cell line, a kind of human leukemia cell.85
For better control of the spontaneous deposition properties, we further used the coprecipitation method to load the microcapsules with polyelectrolytes.89,90,105–107 Quantification of the anti-cancer drugs of daunorubicin (DNR) and DOX loading was performed under different conditions, revealing that a larger amount of drugs could be incorporated at higher drug feeding concentrations and higher salt concentrations.105,106 They showed a strong ability to accumulate the positively charged DOX with a factor of tens to hundreds, that is, the drug concentration within the microcapsules was hundreds of times higher than the feeding concentration. The drug release behaviors from the microcapsules with different layer numbers were studied too, revealing a diffusion controlled release mechanism at the initial stage (4 h). Then application of the drug loaded capsules for tumor treatment was demonstrated by in vitrocell culture and in vivo animal experiments. The in vitro experiments showed that the encapsulated drug can effectively induce the apoptosis of HepG2 tumor cells. By seeding the HepG2 hepatoma cells into nude mice, tumors were created for the experimental studies. The results showed that the encapsulated DOX has better efficacy than that of the free drug in terms of tumor inhibition in a 4 week in vivo culture period.107
Besides from the capsule interiors, release of biologically relevant substances such as DNA, proteins and enzymes from the shells of the capsules and core–shell particles can also be achieved by disassembly or degradation of the shells, such as presented by Donath and co-workers.108 They coated silica particles with protamine and dextran sulfate multilayers, in which plasmid DNAs were embedded. The particles could be taken up by HEK 293 T cells, and then the multilayers were defoliated to release the DNA, resulting in the expression of plasmid encoded proteins in the cells. An interesting point is that different plasmids can be incorporated to a different depth in the multilayer shells, thus co-expression of different proteins in a controlled manner is possible. Moreover, the tailor-made LbL capsules or core–shell particles may have both protective function and targeted delivery properties. In a later work,109 the same group used the capsules encapsulated with pH sensitive fluorescent polymer conjugates to report the intracellular environment pH of the internalized colloidal particles. They identified endosomal escape as the bottleneck limiting the efficacy of the transfection.
3.2 Surface modification for stealth function or targeting
Surface properties greatly influence the performance of microcapsules in the biological environment. Thus considerable efforts have been given to surface modification of microcapsules and multilayer coated colloids so that they will not be recognized by the hosts as foreign materials or they can be targeted to specific sites or cells. More details on fabrication of LbL capsules with biocompatible surfaces or decorated with biofunctional molecules can be found in recent reviews.21,26 Below we mainly summarize the more recent progress in this direction.It has been verified that the assembly of a final monolayer of polycationic copolymer poly(L-lysine)-graft-poly(ethylene glycol) (PLL-g-PEG) onto the PSS-terminated multilayers can impart protein resistance to the surface.110 The quality of the PLL-g-PEG layer, which greatly influences the protein resistance of the surface, on the LbL microcapsules depends on both the type of core material and the dissolution protocols used. The best protein resistance is achieved by using poly(lactic acid) (PLA) cores and coating the microcapsules with PLL-g-PEG after core removal. If the top copolymer layer contains a fraction of the PEG chains end-functionalized with biotin, it may further specifically recognize and immobilize controlled amounts of streptavidin on the capsule surfaces. These capsules have potential applications as novel platforms for biotechnological applications such as biosensors and carriers for targeted drug delivery. One of the other important requirements for biomedical applications is the escape of clearance by the mononuclear phagocytic system (MPS). Merkle and co-workers further studied the effects of PLL-g-PEG and poly-L-glutamic acid (PGA)-g-PEG coating on the PAH/PSS capsules in terms of MPS recognition through in vitrocell culture of human monocyte derived dendritic cells and macrophages.111 It was found that the PGA-g-PEG coating had no significant effect on the cellular recognition, possibly due to the insufficient PEG density. In contrast, PLL-g-PEG effectively blocked the phagocytosis of the coated microcapsules (Fig. 9).
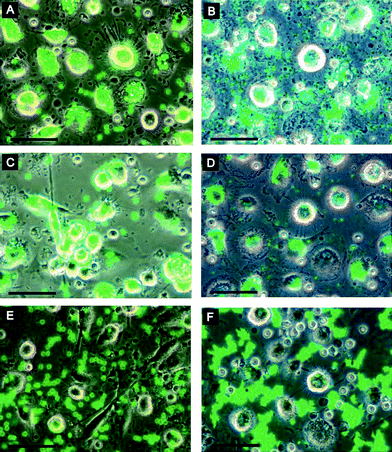 | ||
| Fig. 9 Phagocytosis of coated microcapsules: dendritic cells (left) and macrophages (right) were incubated with 4.8 μm (left) or 1.2 μm (right) microcapsules for 4 h. Images were obtained by an overlay of phase contrast and fluorescence channels. (A, B) PSS-coated microcapsules; (C, D) PLL-coated microcapsules; (E, F) PLL-g-PEG coated microcapsules. Scale bars = 50μm. Reprinted with permission from ref. 111. Copyright 2008, American Chemical Society. | ||
Nowadays great interest has been attracted by integration specific biofunctionality onto the capsule surface for targeting. The concept has been demonstrated recently by targeting the microcapsules on a patterned surface by electrostatic interaction112–114 or recognition between biotin and avidin.115 By microcontact printing, patterns of oppositely charged polyelectrolytes to the top layer of the substrate multilayers were created, which were then used to attract the microcapsules by electrostatic interaction, forming capsule arrays.114 Moreover, a method that allows isolation of the individual capsules and the patterning assembly with satisfactory spatial selectivity is developed via avidin–biotin recognition (Fig. 10).115 The stable microcapsule arrays were further utilized as microreactors to synthesize quantum dots (QDs) and other nanoparticles. Release of the spatially synthesized products can be tuned easily as well (Fig. 10). Donath and co-workers recently demonstrated that the phosphatidylserine coated LbL core–shell microparticles or capsules fused with rubella-like particles (RLPs) can facilitate the membrane passage (Fig. 11).116 On incubation of the lipid-coated LbL colloids with RLPs at low pH (pH 4), the particles attached to the lipid layer by electrostatic forces and subsequently fused with the membrane, presenting the virus envelope proteins on the particle surface. The key function of the virus surface is the binding to a host cell surface, induction of endocytosis, and subsequent fusion with the endosome membranes. Cell culture results confirmed that the particles integrated with RLPs can retain their biological activity and enhance the cell membrane passage.
 | ||
| Fig. 10 (a) Schematic illustration to show the strategy and bio-affinity force for capsule patterning. Avidin molecules are covalently patterned on a polymer film having pentafluorophenyl esters by microcontact printing, which are used to guide the spatial location of biotinylated capsules. Polymers with chelating groups (exemplified with PVA here) are loaded in the capsules to facilitate precipitation or reduction reactions to synthesize QDs, nanocrystals and nanoparticles (illustrated by the production of the ZnS QDs). (b) Fluorescence image to show the existence of ZnS QDs formed exclusively within the aligned microcapsules. (c) Excitation and emission spectra of aligned microcapsules containing ZnS QDs. (d) The evolution of the fluorescence emission at 470 nm as a function of time. The solid and the dashed lines represent the fluorescence intensity from the as-prepared QDs containing capsule arrays and aligned QDs containing capsules having additional (PSS/PAH)3 layers, respectively. Insets show the fluorescence images of capsule patterns after incubation in buffer for 36 d. The scale bars in (b) and (d) represent 30 μm and 20 μm, respectively. Reprinted with permission from ref. 115. Copyright 2006, Wiley-VCH. | ||
 | ||
| Fig. 11 Protocol for engineering virus functionality on polyelectrolyte lipid composite colloids and capsules. Removal of the core is optional. Reprinted with permission from ref. 116. Copyright 2005, Wiley-VCH. | ||
Targeting and uptake of specific antibody modified LbL particles and capsules to colorectal cancer cells were recently demonstrated by Caruso and co-workers.117 They immobilized the humanized A33 monoclonal antibody (HuA33 mAb) which can bind to a transmembrane glycoprotein, the human A33 antigen, on the LbL particles and capsules through electrostatic interaction. A33 antigen is expressed by 95% of human colorectal tumor cells as well as on the basolateral surfaces of intestinal epithelial cells, but not by other epithelial tissues. The cell culture experiments confirmed that the HuA33 mAb decorated particles can be targeted and selectively internalized by colorectal tumor cells and the binding was found through antibody–antigen specific interaction.
3.3 Biosensors
Because of the diverse loading methods for assay elements and easy functionalization, the LbL microcapsules and microparticles are one of the ideal platforms for sensing applications. McShane’s group contributes significantly to this area. In their earlier work,118 the capsules were loaded with a model fluorescent assay consisting of a sodium-sensitive dye and a reference fluorophore. The capsules are mechanically robust, and the encapsulated fluorophores are well retained. Results from sodium sensitivity experiments suggest that the capsules have an excellent potential for use as sensors, with a highly linear response over a broad range of concentrations (0–100 mM). Later on, an oxygen sensor was designed using a similar strategy.119 Besides the fluorescent assay, the microcapsules are also attractive for encapsulating competitive binding assays that require free movement of molecules inside the capsules.120Hydrogels have several unique properties that facilitate the loading of a variety of enzymes and dyes for chemical sensing. However, control over release rates or stable encapsulation is still a challenge. Recently the McShane group designed a glucose sensor by encapsulation of glucose oxidase (GOD) and an oxygen-quenched ruthenium compound within calcium alginate microspheres followed by multilayer coating.121 The multilayer films were used to stabilize the entrapped enzyme and control substrate diffusion. It was demonstrated that the application of multilayer thin films to the alginate microspheres was effective in reducing the leaching rate and total loss of the encapsulated materials from the microspheres.121,122 The polyelectrolyte pairs for assembly, layer number and crosslinking all have great influences on the loss rate of the encapsulated enzyme and the enzyme activity for long term storage.123,124 These findings demonstrate that enzyme immobilization and stabilization can be achieved by using simple modifications to the LbL assembly technique.
The LbL multilayer microcapsules also can work as mobile pH-sensors to monitor the local pH inside living cells (Fig. 12).125 The microcapsules were loaded with a pH sensitive, high molecular weight SNARF-1-dextran conjugate. SNARF-1 exhibits a significant pH-dependent emission shift from green to red fluorescence under acidic and basic conditions, respectively. Its unique spectral properties are maintained after the encapsulation as well. Therefore, the pH change of the local environments of the SNARF-1-filled capsules can be monitored during the transition from the alkaline cell culture medium to the acidic endosomal/lysosomal compartments of human breast cancer cells and fibroblasts.
 | ||
| Fig. 12 SNARF-loaded capsules change from red to green fluorescence upon internalization by MDA-MB435S breast cancer cells. (A) SNARF-fluorescence after adding the capsules to the cell culture and 30 min equilibration. Most of the capsules are outside of the cells and exhibit red fluorescence due to the alkaline pH of the medium. (B) The same cells after another 30 min of incubation. Capsules remaining in the cell medium retain their red fluorescence (red arrows). Capsules that were already incorporated in the acidic endosome in the first image retain their green fluorescence (green arrows). Some capsules were incorporated in endosomal/lysosomal compartments inside cells within the period of 30 min, which is indicated by their change in fluorescence from red to green (red to green arrows). Both images comprise an overlay of microscopy images obtained with phase contrast, a red and a green filter set. (C) Schematic presentation of the endocytotic capsule uptake. Reprinted with permission from ref. 125. Copyright 2007, Royal Society of Chemistry. | ||
The multilayer capsules loaded with low molecular weight fluorescent compounds have also been utilized in immunoassays, which has 70–2000 fold higher sensitivity than that of the corresponding immunoassay performed with direct fluorescently labeled antibodies.126 Donath and co-workers recently used the LbL approach to obtain microbeads coated with authentic viral surfaces, which can catch and quantify virus-specific antibodies in a flow cytometric analysis.127 The antigenic surfaces were generated by fusion of multiple virus subtypes to a lipid bilayer, which surrounds the multilayer coated particles. The multilayer thin film can even be used to coat the living mammalian cells as a protective shell, which allows free diffusion of small molecules, but prevents internalization of proteins larger than 60 kDa into the cells.128 The best cell survival after coating is as high as >80% and the coated cells can be used as biosensors to detect specific compounds.
3.4 Bioreactors
 | ||
| Fig. 13 Optical microscopy images of CaCO3growth at different stages. Reprinted with permission from ref. 129. Copyright 2003, Wiley-VCH. | ||
LbL technology is especially convenient for fabricating complex structures, such as shell-in-shell structures.135 Recently, LbL microcapsules with a shell-in-shell structure were fabricated for integrated and spatially confined enzymatic reactions (Fig. 14).136 The strategy involves the fabrication of spherical ball-in-ball particles consisting of two concentric CaCO3 compartments that can be independently loaded with macromolecules. These two compartments are separated by the polyelectrolyte multilayers. By further deposition of the polyelectrolyte multilayers on the surfaces of ball-in-ball particles followed by decomposition of the CaCO3 particles, novel shell-in-shell microcapsules containing biomacromolecules separated by semipermeable membranes can be obtained. GOD and POD were encapsulated in the outer and inner compartments, respectively. The coupling of both enzymatic reactions could be mediated by the semipermeable character of the polyelectrolyte shells, which allow small substrate (and product) molecules to diffuse in and out, whilst confining the enzyme molecules in the isolated compartments. When glucose is added, H2O2 is generated in the outer compartment, and diffuses into both the bulk solution and the inner compartment. When amplex red is added, resorufin is only formed in the inner compartment as both POD and H2O2 are required. One can observe that the fluorescence emission from the resorufin appeared within a few seconds in the inner compartment, which then spread into the outer compartment of the shell-in-shell structure (Fig. 15). Such two compartment capsules exhibit exciting potential especially for biomedical reactions in a confined space. The barriers between the integrated components can be remotely removed, thus enabling mixing and, hence, the start of a reaction by an external trigger. This was demonstrated later by the same group.137 Light absorbing gold nanoparticles were incorporated into the inner shells. The intermixing of the contents of the two compartments occurred when the inner shells were disrupted by a near-infrared laser light illumination.94–98
 | ||
| Fig. 14 General route for the synthesis of shell-in-shell microcapsules. A = initial core; B = core–shell particle; C = ball-in-ball particle (type I); D = ball-in-ball particle (type II); E = shell-in-shell microcapsule. Reprinted with permission from ref. 136. Copyright 2007, Wiley-VCH. | ||
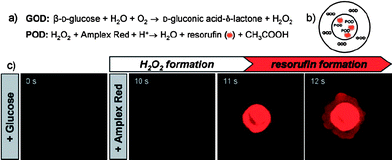 | ||
| Fig. 15 Coupled enzymatic test using GOD and POD inside shell-in-shell capsules. (a) Reaction schemes. (b) Localization of GOD and POD within shell-in-shell capsules (c) CLSMin situ imaging of resorufin formation. Reprinted with permission from ref. 136. Copyright 2007, Wiley-VCH. | ||
 | ||
| Fig. 16 Schematic representation of the arrangement of CF0F1ATPase in lipid-coated microcapsules. ADP: adenosine diphosphate; Pi: inorganic phosphate. Reprinted with permission from ref. 143. Copyright 2007, Wiley-VCH. | ||
4. Conclusions and outlooks
The LbL assembled multilayer microcapsules present a class of advanced materials and have potential applications in many areas of science and technology, owing to their tailorable structures, compositions and properties, and the ease of property manipulation and functionalization. In particular, recent attentions has been paid to the multilayer microcapsules assembled based on new driving forces, and the integration of biofunctional moieties into the microcapsules aiming at diverse bio-related applications, as illustrated in this review.Although a lot of significant achievements have been made in this area in the past decade, there are still some challenges that need to be fulfilled. First, it is important to develop new strategies for the fabrication of LbL microcapsules or manipulation of their properties, with special purposes for functionalization and thereby applications. Different strategies can bring substantial differences to the microcapsules in terms of their chemical and physical structures, stability and stimuli-response, and inevitable their functionality and applicability. Moreover, former basic scientific researches on LbL capsules, most of which are based on electrostatic interaction and hydrogen bonding, focus on understanding their physicochemical properties, such as permeability and mechanical properties. Now works on mimicking the functionalities of cells such as biochemical reactions in a confined space and selective ion transportation are emerging, yet still more efforts should be given to this direction. For example, the LbL microcapsules mimicking the deformability of red blood cells by shape design and interior and surface engineering would be very helpful for drug delivery. Finally, drug delivery vehicles are one of the most possible applications of the LbL microcapsule so far, as revealed by those many existing studies. However, in our opinion, there are still many challenges remaining, including the application of cheaper raw materials and improvement of the fabrication efficiency, optimization of the drug release profile for a specific therapy or disease treatment, the integration of biofunctional moieties onto the microcapsule surfaces aiming at target delivery, and biocompatibility in both the tissue and blood contacting levels. For example, when the drug loaded microcapsules are used by a manner of vein injection, the microcapsule system must possess good enough blood compatibility for purposes of long term circulation and target delivery. For other applications, both the capsule structure and performance should be designed and controlled according to the requirements of the exact situation, and can compete with other existing methods. We believe these challenges will be addressed sooner or later, and can be expedited by collaborations of multidisciplinary investigators from chemistry, pharmaceutics, biology, material science as well as medicine.
Acknowledgements
We appreciate the financial support of the China Postdoctoral Science Foundation (No. 20070421171), Natural Science Foundation of China (No. 20434030 and 20774084) and the National Science Fund for Distinguished Young Scholars of China (No. 50425311).References
- Hollow and Solid Spheres and Microspheres: Science and Technology Associated With Their Fabrication and Application, ed. D. L. Wilcox, M. Berg, T. Bernat, D. Kellerman and J. K. Cochran, Materials Research Society, Warrendale, PA, 1995 Search PubMed.
- G. Decher, J.-D. Hong and J. Schmitt, Macromol. Chem., Macromol. Symp., 1991, 46, 321 Search PubMed.
- G. Decher, Science, 1997, 277, 1232 CrossRef CAS.
- G. B. Sukhorukov, E. Donath, H. Lichtenfeld, E. Knippel, M. Knippel, A. Budde and H. Möhwald, Colloids Surf., A, 1998, 137, 253 CrossRef.
- E. Donath, G. B. Sukhorukov, F. Caruso, S. A. Davis and H. Möhwald, Angew. Chem., Int. Ed., 1998, 37, 2202 CrossRef.
- F. Caruso, R. A. Caruso and H. Möhwald, Science, 1998, 282, 1111 CrossRef CAS.
- C. S. Peyratout and L. Dähne, Angew. Chem., Int. Ed., 2004, 43, 3762 CrossRef.
- C. Schuler and F. Caruso, Biomacromolecules, 2001, 2, 921 CrossRef CAS.
- S. Moya, E. Donath, G. B. Sukhorukov, M. Auch, H. Baumler, H. Lichtenfeld and H. Möhwald, Macromolecules, 2000, 33, 4538 CrossRef CAS.
- G. B. Sukhorukov, L. Dähne, J. Hartmann, E. Donath and H. Möhwald, Adv. Mater., 2000, 12, 112 CrossRef CAS.
- Z. F. Dai, L. Dähne, H. Möhwald and B. Tiersch, Angew. Chem., Int. Ed., 2002, 41, 4019 CrossRef CAS.
- I. L. Radtchenko, G. B. Sukhorukov, S. Leporatti, G. B. Khomutov, E. Donath and H. Möhwald, J. Colloid Interface Sci., 2000, 230, 272 CrossRef CAS.
- G. B. Sukhorukov, A. L. Rogach, B. Zebli, T. Liedl, A. G. Skirtach, K. Kohler, A. A. Antipov, N. Gaponik, A. S. Susha, M. Winterhalter and W. J. Parak, Small, 2005, 1, 194 CrossRef CAS.
- G. B. Sukhorukov, A. L. Rogach, M. Garstka, S. Springer, W. J. Parak, A. Munoz-Javier, O. Kreft, A. G. Skirtach, A. S. Susha, Y. Ramaye, R. Palankar and M. Winterhalter, Small, 2007, 3, 944 CrossRef CAS.
- G. B. Sukhorukov and H. Möhwald, Trends Biotechnol., 2007, 25, 93 CrossRef CAS.
- S. A. Sukhishvili, Curr. Opin. Colloid Interface Sci., 2005, 10, 37 CrossRef CAS.
- A. A. Antipov and G. B. Sukhorukov, Adv. Colloid Interface Sci., 2004, 111, 49 CrossRef CAS.
- G. B. Sukhorukov, A. Fery, M. Brumen and H. Möhwald, Phys. Chem. Chem. Phys., 2004, 6, 4078 RSC.
- G. B. Sukhorukov, A. Fery and H. Möhwald, Prog. Polym. Sci., 2005, 30, 885 CrossRef CAS.
- B. G. De Geest, N. N. Sanders, G. B. Sukhorukov, J. Demeester and S. C. De Smedt, Chem. Soc. Rev., 2007, 36, 636 RSC.
- A. P. R. Johnston, C. Cortez, A. S. Angelatos and F. Caruso, Curr. Opin. Colloid Interface Sci., 2006, 11, 203 CrossRef CAS.
- D. G. Shchukin and G. B. Sukhorukov, Adv. Mater., 2004, 16, 671 CrossRef CAS.
- A. Fery, F. Dubreuil and H. Möhwald, New J. Phys., 2004, 6, 18 CrossRef.
- O. I. Vinogradova, J. Phys.: Condens. Matter, 2004, 16, R1105 CrossRef CAS.
- O. I. Vinogradova, O. V. Lebedeva and B. S. Kim, Annu. Rev. Mater. Res., 2006, 36, 143 CrossRef CAS.
- A. S. Angelatos, K. Katagiri and F. Caruso, Soft Matter, 2006, 2, 18 RSC.
- J. B. Li, H. Möhwald, Z. H. An and G. Lu, Soft Matter, 2005, 1, 259 RSC.
- J. J. Harris, P. M. DeRose and M. L. Bruening, J. Am. Chem. Soc., 1999, 121, 1978 CrossRef CAS.
- J. Q. Sun, T. Wu, Y. P. Sun, Z. Q. Wang, X. Zhang, J. C. Shen and W. X. Cao, Chem. Commun., 1998, 17, 1853 Search PubMed.
- W. J. Tong and C. Y. Gao, Polym. Adv. Technol., 2005, 16, 827 CrossRef CAS.
- C. Gao, E. Donath, S. Moya, V. Dudnik and H. Möhwald, Eur. Phys. J. E, 2001, 5, 21 CrossRef CAS.
- C. Y. Gao, S. Leporatti, S. Moya, E. Donath and H. Möhwald, Langmuir, 2001, 17, 3491 CrossRef CAS.
- I. Pastoriza-Santos, B. Scholer and F. Caruso, Adv. Funct. Mater., 2001, 11, 122 CrossRef CAS.
- H. G. Zhu and M. J. McShane, Langmuir, 2005, 21, 424 CrossRef CAS.
- P. Schuetz and F. Caruso, Adv. Funct. Mater., 2003, 13, 929 CrossRef CAS.
- T. Mauser, C. Dejugnat and G. B. Sukhorukov, Macromol. Rapid Commun., 2004, 25, 1781 CrossRef CAS.
- W. J. Tong, C. Y. Gao and H. Möhwald, Chem. Mater., 2005, 17, 4610 CrossRef CAS.
- W. J. Tong, C. Y. Gao and H. Möhwald, Macromolecules, 2006, 39, 335 CrossRef CAS.
- J. F. Quinn, A. P. R. Johnston, G. K. Such, A. N. Zelikin and F. Caruso, Chem. Soc. Rev., 2007, 36, 707 RSC.
- S. A. Sukhishvili and S. Granick, J. Am. Chem. Soc., 2000, 122, 9550 CrossRef CAS.
- S. A. Sukhishvili and S. Granick, Macromolecules, 2002, 35, 301 CrossRef CAS.
- S. Y. Yang and M. F. Rubner, J. Am. Chem. Soc., 2002, 124, 2100 CrossRef CAS.
- S. Y. Yang, D. Lee, R. E. Cohen and M. F. Rubner, Langmuir, 2004, 20, 5978 CrossRef CAS.
- V. Kozlovskaya, S. Ok, A. Sousa, M. Libera and S. A. Sukhishvili, Macromolecules, 2003, 36, 8590 CrossRef CAS.
- V. Kozlovskaya, E. Kharlampieva, M. L. Mansfield and S. A. Sukhishvili, Chem. Mater., 2006, 18, 328 CrossRef CAS.
- V. Kozlovskaya and S. A. Sukhishvili, Macromolecules, 2006, 39, 6191 CrossRef CAS.
- A. N. Zelikin, J. F. Quinn and F. Caruso, Biomacromolecules, 2006, 7, 27 CrossRef CAS.
- A. N. Zelikin, Q. Li and F. Caruso, Angew. Chem., Int. Ed., 2006, 45, 7743 CrossRef CAS.
- Y. J. Zhang, S. G. Yang, Y. Guan, W. X. Cao and J. Xu, Macromolecules, 2003, 36, 4238 CrossRef CAS.
- Z. Q. Feng, Z. P. Wang, C. Y. Gao and J. C. Shen, Adv. Mater., 2007, 19, 3687 CrossRef CAS.
- W. J. Tong, C. Y. Gao and H. Möhwald, Macromol. Rapid Commun., 2006, 27, 2078 CrossRef CAS.
- W. J. Tong, C. Y. Gao and H. Möhwald, Polym. Adv. Technol., 2008, 19, 817 CrossRef.
- G. K. Such, E. Tjipto, A. Postma, A. P. R. Johnston and F. Caruso, Nano Lett., 2007, 7, 1706 CrossRef CAS.
- G. K. Such, J. F. Quinn, A. Quinn, E. Tjipto and F. Caruso, J. Am. Chem. Soc., 2006, 128, 9318 CrossRef CAS.
- J. M. Lehn, Angew. Chem., Int. Ed. Engl., 1990, 29, 1304 CrossRef.
- J. M. Lehn, Science, 1993, 260, 1762 CrossRef CAS.
- J. M. Lehn, Supramolecular Chemistry: Concepts and Perspectives, VCH, Weinheim, 1995, ch. 1 Search PubMed.
- O. Crespo-Biel, B. Dordi, D. N. Reinhoudt and J. Huskens, J. Am. Chem. Soc., 2005, 127, 7594 CrossRef CAS.
- A. Van der Heyden, M. Wilczewski, P. Labbé and R. Auzély, Chem. Commun., 2006, 30, 3220 Search PubMed.
- I. Suzuki, Y. Egawa, Y. Mizukawa, T. Hoshi and J. Anzai, Chem. Commun., 2002, 2, 164 Search PubMed.
- Z. P. Wang, Z. Q. Feng and C. Y. Gao, Chem. Mater., 2008 Search PubMed , DOI: 10.1021/cm8003358.
- A. P. R. Johnston, E. S. Read and F. Caruso, Nano Lett., 2005, 5, 953 CrossRef CAS.
- A. P. R. Johnston, H. Mitomo, E. S. Read and F. Caruso, Langmuir, 2006, 22, 3251 CrossRef CAS.
- A. P. R. Johnston and F. Caruso, Angew. Chem., Int. Ed., 2007, 46, 2677 CrossRef CAS.
- C. Y. Gao, H. Möhwald and J. C. Shen, ChemPhysChem, 2004, 5, 116 CrossRef CAS.
- T. Serizawa, K. Hamada, T. Kitayama, N. Fujimoto, K. Hatada and M. Akashi, J. Am. Chem. Soc., 2000, 122, 1891 CrossRef CAS.
- T. Kida, M. Mouri and M. Akashi, Angew. Chem., Int. Ed., 2006, 45, 7534 CrossRef CAS.
- G. Ibarz, L. Dähne, E. Donath and H. Möhwald, Adv. Mater., 2001, 13, 1324 CrossRef CAS.
- A. A. Antipov, G. B. Sukhorukov, S. Leporatti, I. L. Radtchenko, E. Donath and H. Möhwald, Colloids Surf., A, 2002, 198, 535 CrossRef.
- G. B. Sukhorukov, A. A. Antipov, A. Voigt, E. Donath and H. Möhwald, Macromol. Rapid Comm., 2001, 22, 44 CrossRef CAS.
- Y. Lvov, A. A. Antipov, A. Mamedov, H. Möhwald and G. B. Sukhorukov, Nano Lett., 2001, 1, 125 CrossRef CAS.
- K. Köhler and G. B. Sukhorukov, Adv. Funct. Mater., 2007, 17, 2053 CrossRef.
- F. Caruso, D. Trau, H. Möhwald and R. Renneberg, Langmuir, 2000, 16, 1485 CrossRef CAS.
- F. Caruso, W. J. Yang, D. Trau and R. Renneberg, Langmuir, 2000, 16, 8932 CrossRef CAS.
- X. Y. Shi and F. Caruso, Langmuir, 2001, 17, 2036 CrossRef CAS.
- X. P. Qiu, S. Leporatti, E. Donath and H. Möhwald, Langmuir, 2001, 17, 5375 CrossRef CAS.
- M. E. Bobreshova, G. B. Sukhorukov, E. A. Saburova, L. I. Elfimova, L. I. Shabarchina and B. I. Sukhorukov, Biofizika, 1999, 44, 813 CAS.
- N. G. Balabushevitch, G. B. Sukhorukov, N. A. Moroz, D. V. Volodkin, N. I. Larionova, E. Donath and H. Möhwald, Biotechnol. Bioeng., 2001, 76, 207 CrossRef CAS.
- Y. J. Wang and F. Caruso, Chem. Commun., 2004, 13, 1528 Search PubMed.
- D. V. Volodkin, N. I. Larionova and G. B. Sukhorukov, Biomacromolecules, 2004, 5, 1962 CrossRef CAS.
- Y. J. Wang, A. M. Yu and F. Caruso, Angew. Chem., Int. Ed., 2005, 44, 2888 CrossRef CAS.
- Y. J. Wang and F. Caruso, Chem. Mater., 2005, 17, 953 CrossRef CAS.
- I. L. Radtchenko, G. B. Sukhorukov and H. Möhwald, Colloids Surf., A, 2002, 202, 127 CrossRef CAS.
- C. Y. Gao, E. Donath, H. Möhwald and J. C. Shen, Angew. Chem., Int. Ed., 2002, 41, 3789 CrossRef CAS.
- X. Y. Liu, C. Y. Gao, J. C. Shen and H. Möhwald, Macromol. Biosci., 2005, 5, 1209 CrossRef CAS.
- A. J. Khopade and F. Caruso, Biomacromolecules, 2002, 3, 1154 CrossRef CAS.
- A. I. Petrov, D. V. Volodkin and G. B. Sukhorukov, Biotechnol. Prog., 2005, 21, 918 CrossRef CAS.
- C. Y. Gao, X. Y. Liu, J. C. Shen and H. Möhwald, Chem. Commun., 2002, 17, 1928 Search PubMed.
- W. J. Tong, W. F. Dong, C. Y. Gao and H. Möhwald, J. Phys. Chem. B, 2005, 109, 13159 CrossRef CAS.
- W. J. Tong, H. Q. Song, C. Y. Gao and H. Möhwald, J. Phys. Chem. B, 2006, 110, 12905 CrossRef CAS.
- T. Borodina, E. Markvicheva, S. Kunizhev, H. Moehwald, G. B. Sukhorukov and O. Kreft, Macromol. Rapid Commun., 2007, 28, 1894 CrossRef CAS.
- B. G. De Geest, R. E. Vandenbroucke, A. M. Guenther, G. B. Sukhorukov, W. E. Hennink, N. N. Sanders, J. Demeester and S. C. De Smedt, Adv. Mater., 2006, 18, 1005 CrossRef.
- X. D. Li, Q. L. Hu, L. H. Yue and J. C. Shen, Chem.–Eur. J., 2006, 12, 5770 CrossRef CAS.
- A. G. Skirtach, A. A. Antipov, D. G. Shchukin and G. B. Sukhorukov, Langmuir, 2004, 20, 6988 CrossRef CAS.
- A. S. Angelatos, B. Radt and F. Caruso, J. Phys. Chem. B, 2005, 109, 3071 CrossRef CAS.
- B. Radt, T. A. Smith and F. Caruso, Adv. Mater., 2004, 16, 2184 CrossRef CAS.
- A. G. Skirtach, C. Dejugnat, D. Braun, A. S. Susha, A. L. Rogach, W. J. Parak, H. Möhwald and G. B. Sukhorukov, Nano Lett., 2005, 5, 1371 CrossRef CAS.
- A. G. Skirtach, A. M. Javier, O. Kreft, K. Kohler, A. P. Alberola, H. Möhwald, W. J. Parak and G. B. Sukhorukov, Angew. Chem., Int. Ed., 2006, 45, 4612 CrossRef CAS.
- D. G. Shchukin, D. A. Gorin and H. Moehwald, Langmuir, 2006, 22, 7400 CrossRef CAS.
- B. G. De Geest, A. G. Skirtach, A. A. Mamedov, A. A. Antipov, N. A. Kotov, S. C. De Smedt and G. B. Sukhorukov, Small, 2007, 3, 804 CrossRef CAS.
- B. G. De Geest, A. M. Jonas, J. Demeester and S. C. De Smedt, Langmuir, 2006, 22, 5070 CrossRef.
- B. G. De Geest, C. Dejugnat, G. B. Sukhorukov, K. Braeckmans, S. C. De Smedt and J. Demeester, Adv. Mater., 2005, 17, 2357 CrossRef CAS.
- Y. Itoh, M. Matsusaki, T. Kida and M. Akashi, Biomacromolecules, 2006, 7, 2715 CrossRef CAS.
- Z. W. Mao, L. Ma, C. Y. Gao and J. C. Shen, J. Controlled Release, 2005, 104, 193 CrossRef CAS.
- Q. H. Zhao, S. A. Zhang, W. J. Tong, C. Y. Gao and J. C. Shen, Eur. Polym. J., 2006, 42, 3341 CrossRef CAS.
- Q. H. Zhao, Z. W. Mao, C. Y. Gao and J. C. Shen, J. Biomater. Sci., Polym. Ed., 2006, 17, 997 CrossRef CAS.
- Q. H. Zhao, B. S. Han, Z. H. Wang, C. Y. Gao, C. H. Peng and J. C. Shen, Nanomed. Nanotechnol. Biol. Med., 2007, 3, 63 CrossRef CAS.
- U. Reibetanz, C. Claus, E. Typlt, J. Hofmann and E. Donath, Macromol. Biosci., 2006, 6, 153 CrossRef CAS.
- U. Reibetanz, D. Halozan, M. Brumen and E. Donath, Biomacromolecules, 2007, 8, 1927 CrossRef CAS.
- R. Heuberger, G. Sukhorukov, J. Voros, M. Textor and H. Möhwald, Adv. Funct. Mater., 2005, 15, 357 CrossRef CAS.
- U. Wattendorf, O. Kreft, M. Textor, G. B. Sukhorukov and H. P. Merkle, Biomacromolecules, 2008, 9, 100 CrossRef CAS.
- M. Nolte and A. Fery, Langmuir, 2004, 20, 2995 CrossRef CAS.
- M. Nolte and A. Fery, IEEE Trans. Nanobiosci., 2004, 3, 22 CrossRef.
- J. Feng, B. Wang, C. Y. Gao and J. C. Shen, Adv. Mater., 2004, 16, 1940 CrossRef CAS.
- B. Wang, Q. H. Zhao, F. Wang and C. Y. Gao, Angew. Chem., Int. Ed., 2006, 45, 1560 CrossRef CAS.
- M. Fischlechner, O. Zschornig, J. Hofmann and E. Donath, Angew. Chem., Int. Ed., 2005, 44, 2892 CrossRef CAS.
- C. Cortez, E. Tomaskovic-Crook, A. P. R. Johnston, B. Radt, S. H. Cody, A. M. Scott, E. C. Nice, J. K. Heath and F. Caruso, Adv. Mater., 2006, 18, 1998 CrossRef CAS.
- T. A. Duchesne, J. Q. Brown, K. B. Guice, Y. M. Lvov and M. J. McShane, Sens. Mater., 2002, 14, 293 Search PubMed.
- M. J. McShane, J. Q. Brown, K. B. Guice and Y. M. Lvov, J. Nanosci. Nanotechnol., 2002, 2, 411 CrossRef CAS.
- S. Chinnayelka and M. J. McShane, Diabetes Technol. Ther., 2006, 8, 269 CrossRef CAS.
- J. Q. Brown, R. Srivastava and M. J. McShane, Biosens. Bioelectron., 2005, 21, 212 CrossRef CAS.
- R. Srivastava and M. J. McShane, J. Microencapsulation, 2005, 22, 397 CrossRef CAS.
- R. Srivastava, J. Q. Brown, H. G. Zhu and M. J. McShane, Macromol. Biosci., 2005, 5, 717 CrossRef CAS.
- R. Srivastava, J. Q. Brown, H. G. Zhu and M. J. McShane, Biotechnol. Bioeng., 2005, 91, 124 CrossRef CAS.
- O. Kreft, A. M. Javier, G. B. Sukhorukov and W. J. Parak, J. Mater. Chem., 2007, 17, 4471 RSC.
- D. Trau, W. J. Yang, M. Seydack, F. Caruso, N. T. Yu and R. Renneberg, Anal. Chem., 2002, 74, 5480 CrossRef CAS.
- L. Toellner, M. Fischlechner, B. Ferko, R. M. Grabherr and E. Donath, Clin. Chem., 2006, 52, 1575 CrossRef CAS.
- M. Germain, P. Balaguer, J. C. Nicolas, F. Lopez, J. P. Esteve, G. B. Sukhorukov, M. Winterhalter, H. Richard-Foy and D. Fournier, Biosens. Bioelectron., 2006, 21, 1566 CrossRef CAS.
- A. Antipov, D. Shchukin, Y. Fedutik, I. Zanaveskina, V. Klechkovskaya, G. Sukhorukov and H. Möhwald, Macromol. Rapid Commun., 2003, 24, 274 CrossRef CAS.
- A. M. Yu, I. Gentle, G. Q. Lu and F. Caruso, Chem. Commun., 2006, 20, 2150 Search PubMed.
- R. Ghan, T. Shutava, A. Patel, V. T. John and Y. Lvov, Macromolecules, 2004, 37, 4519 CrossRef CAS.
- T. Shutava, Z. G. Zheng, V. John and Y. Lvov, Biomacromolecules, 2004, 5, 914 CrossRef CAS.
- N. G. Balabushevich, G. B. Sukhorukov and N. I. Larionova, Macromol. Rapid Commun., 2005, 26, 1168 CrossRef CAS.
- E. W. Stein, D. V. Volodkin, M. J. McShane and G. B. Sukhorukov, Biomacromolecules, 2006, 7, 710 CrossRef CAS.
- Z. F. Dai, H. Möhwald, B. Tiersch and L. Dähne, Langmuir, 2002, 18, 9533 CrossRef CAS.
- O. Kreft, M. Prevot, H. Möhwald and G. B. Sukhorukov, Angew. Chem., Int. Ed., 2007, 46, 5605 CrossRef.
- O. Kreft, A. G. Skirtach, G. B. Sukhorukov and H. Möhwald, Adv. Mater., 2007, 19, 3142 CrossRef CAS.
- L. Q. Ge, H. Möhwald and J. B. Li, ChemPhysChem, 2003, 4, 1351 CrossRef CAS.
- L. Q. Ge, H. Möhwald and J. B. Li, Chem.–Eur. J., 2003, 9, 2589 CrossRef CAS.
- K. Katagiri and F. Caruso, Macromolecules, 2004, 37, 9947 CrossRef CAS.
- K. Katagiri and F. Caruso, Adv. Mater., 2005, 17, 738 CrossRef CAS.
- O. P. Tiourina, I. Radtchenko, G. B. Sukhorukov and H. Möhwald, J. Membr. Biol., 2002, 190, 9 CrossRef CAS.
- L. Duan, Q. He, K. W. Wang, X. H. Yan, Y. Cui, H. Möwald and J. B. Li, Angew. Chem., Int. Ed., 2007, 46, 6996 CrossRef CAS.
- W. Qi, L. Duan, K. W. Wang, X. H. Yan, Y. Cui, Q. He and J. B. Li, Adv. Mater., 2008, 20, 601 CrossRef CAS.
Footnote |
| † This paper is part of a Journal of Materials Chemistry theme issue on Biology in the Service of Materials. Guest editor: Vincent Rotello. |
| This journal is © The Royal Society of Chemistry 2008 |

