An application of AAO template: orderly assembled organic molecules for surface-enhanced Raman scattering
Zhixun
Luo
a,
Aidong
Peng
a,
Hongbing
Fu
a,
Ying
Ma
a,
Jiannian
Yao
*a and
Boon H.
Loo
b
aBeijing National Laboratory for Molecular Science (BNLMS), Institute of Chemistry, Chinese Academy of Sciences, Beijing 100080, P. R. China. E-mail: jnyao@iccas.ac.cn; Fax: +86-10-82616517; Tel: +86-10-82616517
bDepartment of Chemistry, Towson University, Towson, MD 21252, USA
First published on 26th November 2007
Abstract
High-density ordered arrays of core–shell nano-pillars of Ag–perylene were fabricated using an anodic aluminium oxide (AAO) template which was first embedded with the perylene molecules, followed by an electrochemical deposition of Ag. The surface-enhanced Raman scattering (SERS) spectrum obtained from this system showed well-resolved Raman peaks with good signal-to-noise ratios and little fluorescence background. This is in sharp contrast to the SERS of the individual Ag–perylene nanorods removed from the same AAO template, and the SERS of perylene molecules adsorbed on Ag colloidal nanoparticles. In the latter two cases, the SERS spectra consisted of broad and not-so-well-resolved Raman peaks with a strong fluorescence background. It is believed that the orderly assembly of the perylene molecules on the inner walls of the pores of the AAO template along the Ag nano-pillars led to fluorescence quenching. The high-density ordered arrays of Ag nano-pillars brought forth a surface plasmon resonance for the SERS effect. The present AAO template system offers a new substrate for studying SERS of highly fluorescing molecules.
I. Introduction
Surface-enhanced Raman scattering (SERS) is one of the best techniques to study interfacial effects. It has been widely used to study the orientation and behavior of adsorbed molecules on surfaces, and to analyze the interphase tropism, configuration and conformation of biological molecules.1–9 One of the key targets of the SERS technique is to optimize active substrates for the detection and identification of adsorbed molecular species. If the molecules could be assembled in a uniformly ordered orientation near a metal substrate, the SERS signals might be enhanced. Up to now, only a few reports have touched upon the treatment on the assembly of molecules.Recently it was reported that some molecules formed coincident and uniform assembly with head-to-tail J-aggregation10 along the inner walls of the pores of an anodic aluminium oxide (AAO) template. AAO templates with highly ordered nano-pore arrays11–27 have been widely used as templates for the synthesis of ordered arrays of one-dimensional nanostructures which still maintain similar monodispersity as well as the dimensions of the pores of the templates.28–33
Perylene, a polycyclic aromatic hydrocarbon, is an environmentally hazardous substance that can not be detected effectively in the environment.34 Its large fluorescence yield makes it difficult to obtain its Raman signals under normal conditions.35,36 Moreover, perylene is insoluble in water and the organic solvents used to dissolve it often have strong Raman peaks that interfere with the Raman signals of the perylene molecule. Because of these limitations, SERS of perylene has not been reported.
Based on the AAO template, high-density ordered arrays of core–shell nano-pillars of Ag–perylene were fabricated for SERS studies in the present work. A high-quality SERS spectrum of perylene was obtained, which exhibited intense signals with a fluorescence-free background. The SERS signals compare favorably with those from two other substrate systems that we tried, namely, the Ag colloidal nanoparticles system and the Ag–perylene nanorods system, obtained with individual nanorods removed from an AAO template. The results suggest that the coincident and uniform assembly of the perylene molecules along the Ag nanorods led to the fluorescence quenching. In addition, the high-density ordered arrays of nano-pillars brought forth a surface plasmon resonance to achieve the enhancement in the Raman scattering.37
II. Experimental
All chemicals used are of reagent grade. The AAO templates were prepared according to the method described below.16 Aluminium foils (99.999% purity, Institute of Nonferrous Metal, Chinese Academy of Sciences) were first degreased in acetone and then annealed at 350 °C for 2 h. The annealed foils were then etched in a mixture of perchloric acid and ethanol (1 : 4) for several minutes and anodized at 40 V in 0.40 M oxalic acid at 10 °C for 2 h. Thereafter, the surface oxide layer formed was removed in a mixture of phosphoric acid (6 wt%) and chromic acid (1.5 wt%), and the bare aluminium foils were again anodized under the same conditions as before for 2.5 h.The AAO templates were embedded with the perylene molecules by soaking in a chloroform solution of saturated perylene (sublimed, 99.5% purity, Sigma-Aldrich) for 2 minutes, and then air dried for 15 minutes. This process was repeated several times, and the templates were finally dried at 100 °C in a nitrogen atmosphere.
Silver metal was pulse-deposited into the pores of the templates. The electrodeposition was done in a silver nitrate solution with a ladder decreasing voltage method from an initial voltage of 16 V to a final voltage of 8 V for several hours. A saturated CuCl2 solution was used to remove the Al base from the back side, whereas a phosphoric acid solution (6 wt%) was employed to remove the alumina barrier. SERS active high-density ordered arrays of core–shell nano-pillars of Ag–perylene were thus formed. The Ag–perylene nanorods were obtained by dissolving the AAO template frame with 3 M NaOH solution. Typical diameters of the nanorods were 60 nm, and the lengths were several microns.
The Ag colloidal nanoparticles for SERS were prepared by the following method.38 500 mL of a 1 mM AgNO3 solution was heated to boiling, and 10 ml of a 1% sodium citrate aqueous solution was added into it dropwise, with vigorous stirring. The mixed solution was kept boiling for a further 10 min until a greenish grey silver colloidal solution was obtained. Dynamic light scattering (DLS) results showed that the average nanoparticle size was 87 nm.
All AFM images were obtained on a Seiko SPM3800, the SEM images were done on a Hitachi S4300 and the TEM images were done on a JEOL JSM-6301F. The UV-VIS absorption spectrometer was a Perkin Elmer Lambda 35, and the fluorescence instrument was a Hitachi F-4500.
The SERS spectra were collected on a Renishaw H13325 spectrometer, using 514.5 nm excitation, whereas the normal Raman spectra were done on a Bruker Model IFS-66 FT-Raman spectrometer, with 1064 nm excitation at 50 mW and a line resolution of 3 cm−1.
All calculations were carried out using the Gaussian 03 package. Density functional theory at the B3LYP level was employed with Lanl2dz basis sets and optimized geometry of perylene.
III. Results and discussion
A honeycomb structure of anodic porous alumina was obtained and is shown in Fig. 1a. The pores of the template are uniformly arranged in a close-packed hexagonal lattice, and they are continuous and parallel to one another (Fig. 1b). All of these pores have a uniform diameter of about 60 nm. The TEM and AFM pictures of the perylene nanotubes prepared in the pores of an AAO template by the soakage method before Ag was electrodeposited are shown in Fig. 1c and d, respectively.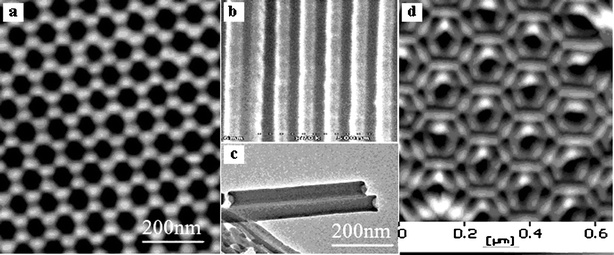 | ||
| Fig. 1 AFM and SEM photos of an ordered AAO template: top-view (a) and cross-section (b); TEM and AFM photos of perylene nanotubes prepared in the pores of AAO templates by the soakage method before Ag was electrodeposited (c, d). | ||
Fig. 2a–c show the AFM images of nano-pillar arrays of Ag–perylene from both the front and back sides of an AAO template. It is obvious that Ag was electrodeposited into close-packed hexagonal lattice spaces (Fig. 2b). Fig. 2d and e show the SEM and AFM images of the Ag–perylene nanorods.
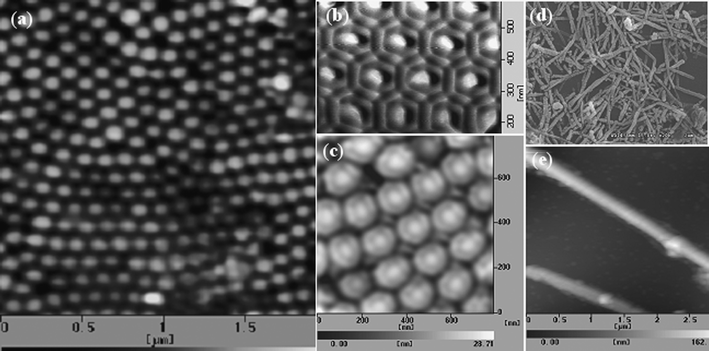 | ||
| Fig. 2 AFM photo of nano-pillar arrays of Ag–perylene from the back side (a); AFM photos of the core–shell structure of Ag–perylene from the front side (b) and back side (c); SEM and AFM photos of Ag–perylene nanorods (d, e). | ||
The UV-VIS experiments were performed to determine whether the perylene molecules would self-assemble on the walls of the pores of the templates after the templates were immersed in a chloroform solution of saturated perylene. The absorption spectrum of 1.2 × 10−6 M perylene in chloroform solution is shown in Fig. 3a whereas the absorption spectrum of the Ag–perylene nanorods is shown in Fig. 3b. Evidently, the absorption profile of the Ag–perylene nanorods shows a great similarity to that of the solution, except for a red shift of the 414 and 438 nm bands and the appearance of an additional band at 483 nm. Hence, the results confirmed the presence of perylene in the AAO template. The embedded perylene molecules formed a J-aggregation due to the appearance of a new band at 483 nm. The J-aggregation is the assembly of the perylene molecules in a head-to-tail arrangement on the walls of the pores of the AAO template.39
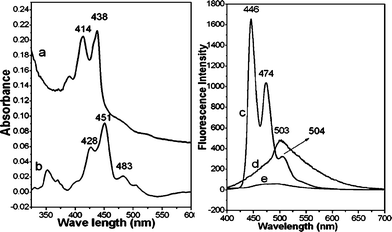 | ||
| Fig. 3 (a) Absorption spectrum of 1.2 × 10−6 M perylene in chloroform solution; (b) absorption spectrum of perylene nanorods, removed from the AAO template with 3 M NaOH solution; (c) fluorescence spectrum of 1.2 × 10−6 M perylene in chloroform solution; (d) fluorescence spectrum of Ag–perylene nanorods; (e) fluorescence spectrum of the Ag–perylene core–shell nano-pillars array. The excitation wavelength for all the fluorescence spectra was 384 nm. | ||
Additional fluorescence experiments also suggest a head-to-tail J-aggregation. The fluorescence spectrum of 1.2 × 10−6 M perylene in chloroform solution consists of three emission bands at 446, 474 and 504 nm (Fig. 3c), attributable to the perylene monomer. However, the fluorescence of the Ag–perylene nanorods shows only a very broad band centered at 503 nm (Fig. 3d). The 503 nm band shows the presence of a J-aggregation. If the perylene molecules were to form an H-aggregation in a face-to-face arrangement, a fluorescence band at a higher wavelength (∼600 nm) would have been observed.40
The fluorescence of perylene was quenched in the presence of the silver metal; Fig. 3e shows the fluorescence spectrum of the Ag–perylene core–shell nano-pillars. The fluorescence was reduced to almost zero, and no bands were visible. Fluorescence quenching suggests a possible Raman enhancement.37 Indeed, strong SERS signals in the region from 200 to 3300 cm−1 with a flat background were observed from the Ag–perylene nano-pillar arrays system (Fig. 4a). The SERS spectrum shows four strong bands at 1292, 1365, 1566 and 1610 cm−1, and they are attributed to the in-plane C–C stretch.41 Several weaker bands, in both the lower- and higher-wavenumber regions, were also observed. In comparison, a poorer quality SERS spectrum was obtained from the individual Ag–perylene nanorods removed from the same nano-pillar array system (Fig. 4b), and from perylene adsorbed on Ag colloidal nanoparticles on a cover glass (Fig. 4c). Three broad bands at 1308, 1388, and 1573 cm−1 were observed in the 1200–1700 cm−1 region. All of these bands sat on a strong fluorescence background. Additionally, spectral features in the C–H stretching region were also lost; only a broad band was observed at ∼2900 cm−1. Therefore, it is concluded that the arrays of Ag core–shell nano-pillars from the AAO template provide a better SERS substrate for molecules that exhibit strong fluorescence. It is significant to find that the SERS spectrum of the Ag–perylene nano-pillar arrays differs remarkably from that of disarrayed Ag–perylene nanorods. This difference necessitates a consideration of the molecular orientation and the surface plasmon effect.42
 | ||
| Fig. 4 SERS spectrum of perylene from the standing Ag–perylene core–shell nano-pillar array (a); SERS spectrum of Ag–perylene nanorods (b); SERS spectrum of perylene on colloidal Ag (c). The excitation wavelength was 514.5 nm. | ||
It is reasoned that the high density ordered arrays of Ag–perylene nano-pillars induce a stronger surface plasmon resonance effect at both tips of the nano-pillars than the Ag nanorods or the Ag colloidal nanoparticles. For a theoretical evaluation of the enhancement mechanism in the nano-pillar system, we begin the consideration with an electromagnetic model where an enhancement factor is given by the equation37,42–47
 | (1) |
The quantity G in eqn (1) can become large as a result of three consequences: (i) the quantity |1 −Γ| becomes small, (ii) the quantity |εQ1(ξ0) −ξ0Q1′(ξ0)| becomes small, or (iii) the quantities ξ0 and ξ1 approach unity, i.e., the ellipsoid becomes needle-like, named as the lightning rod effect.46 If the lightning rod effect is the main cause, the field at the tip is considered as Etip = γEdip + EL, where the quantity  (Aa is the depolarization factor) has the property that γ = 1 for a sphere,37Edip is the dipole field given by Edip = 2μ/a3 and EL is the laser field. So, the SERS signal will depend on Etip4 which can bring forth a giant SERS enhancement.37,47 In fact, a kind of tip array has been demonstrated to be an effective SERS system.48,49
(Aa is the depolarization factor) has the property that γ = 1 for a sphere,37Edip is the dipole field given by Edip = 2μ/a3 and EL is the laser field. So, the SERS signal will depend on Etip4 which can bring forth a giant SERS enhancement.37,47 In fact, a kind of tip array has been demonstrated to be an effective SERS system.48,49
Large enhancements are produced when a substrate absorbs photons and localizes them. Rough surfaces absorb photons and ‘store’ the energy in the surface plasma; the electromagnetic energy is delocalized in the direction parallel to the surface, but localized in the perpendicular direction.37 Here, the high-density ordered array of Ag nano-pillars may present advantages such as an increase in the electromagnetic energy density near the surface; and as the spacing of Ag nano-pillars is close to the excitation wavelength, the local field is increased due to the resonance effect, which in turn causes the dipole moment to increase.
It is insightful to investigate the assembling situation of the perylene molecules. According to the exciton theory of molecules,50–52 different accumulations of molecules have different tilt angles. For the face-to-face (or side-by-side) accumulation, the tilt angle value is greater than 54.7°; whereas for the head-to-tail (or linear) accumulation, it is less than 54.7°. The approximate tilt angle for an accumulation of N molecules can be calculated according to the following equation,53
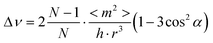 | (2) |
 | ||
| Fig. 5 Top: the head-to-tail assembly of the perylene molecules along the inner wall of an AAO pore with a tilt angle of α after the first dip of the AAO template into a perylene solution. Bottom: the structure of an Ag–perylene nano-pillar assembly after several dips of the AAO template into a perylene solution and electrochemical deposition of Ag metal. | ||
With the first dip of the AAO template into perylene solution, the perylene molecules form a layer along the wall of the pore in a head-to-tail assembly with a tilt angle α. After repeated dips, several layers of the perylene molecules grow into a nanotube. The bottom portion of Fig. 5 shows the structure of an Ag–perylene nano-pillar assembly in which layers of perylene molecules encircle the electrodeposited central Ag rod.
To further verify the Raman activity of perylene, we performed vibrational analysis for the free perylene molecule as well as for perylene adsorbed on Ag. The perylene molecule has a D2h symmetry with a total of 90 normal modes, of which 45 are Raman active modes (g symmetry) and 45 IR active modes (u symmetry).39 Because the molecule has a high fluorescence quantum yield, it is difficult to obtained its normal Raman spectrum under ordinary conditions.34,35,40 We report here the FT-Raman experiments on perylene powder at room temperature. The FT-Raman spectrum of perylene shows many well-resolved peaks and is relatively free of a fluorescence background (Fig. 6b) because the excitation wavelength 1064 nm is far from the absorption bands in the 400 cm−1 region. The normal Raman spectrum compares well with the calculated spectrum for a free perylene molecule (Fig. 6a). The vibrational assignments of the observed bands are given in Table 1.
 | ||
| Fig. 6 (a) Calculated Raman spectrum for a free perylene molecule; (b) normal Raman spectrum of perylene powder excited at 1064 nm; (c) calculated Raman spectrum by assuming that a perylene molecule interacted with an Ag atom; (d) SERS spectrum from the standing Ag–perylene core–shell nano-pillars array. | ||
| SERS of Ag–perylene nano-pillar array | FT-Raman of perylene powder | Calculated Raman activity | Assignment | |
|---|---|---|---|---|
| Raman | SERS | |||
| a vs. = very strong, s. = strong, m. = medium, w. = weak. | ||||
| 352(w.) | 364(m.) | 353(w.) | 354(w.) | in-plane C–C–C bend |
| 544(m.) | 550(w.) | 525(w.) | 527(w.) | in-plane C–C stretch |
| 704(w.) | out-of-plane C–C–C bend | |||
| 784(m.) | 796(w.) | out-of-plane C–C–H bend | ||
| 958(w.) | 979(s.) | 954(m.) | 957(m.) | out-of-plane C–C–H bend |
| 1292(s.) | 1299(s.) | 1287(s.) | 1292(s.) | in-plane C–C stretch |
| 1365(s.) | 1373(s.) | 1383(s.) | 1388(s.) | in-plane C–C stretch |
| 1452(w.) | 1452(w.) | In-plane C–C–H bend | ||
| 1566(s.) | 1570(vs.) | 1555(vs.) | 1560(vs.) | in-plane C–C stretch |
| 1610(m.) | 1622(w.) | 1618(w.) | in-plane C–C stretch | |
There is also a good match between the SERS spectrum of the standing Ag–perylene core–shell nano-pillars array (Fig. 6d) and the normal Raman spectrum of the perylene molecule. For example, the 1292 cm−1 band in Fig. 6d can be correlated with the 1299 cm−1 band in Fig. 6b, the 1366 cm−1 band with the 1373 cm−1 band, the 1566 cm−1 band with the 1570 cm−1 band, and the 1610 cm−1 band with the 1622 cm−1 band. We have also performed a Raman spectrum calculation by assuming a perylene molecule interacted with an Ag atom.54 The calculated spectrum is shown in Fig. 6c. Again, there is a good one to one correspondence between the calculated and the observed SERS bands.
IV. Conclusions
High-quality SERS spectra of perylene from the ordered arrays of Ag–perylene core–shell nano-pillars have been obtained. The SERS from the nano-pillar arrays system shows a sharp contrast to the SERS from the individual Ag–perylene nanorods and the SERS from Ag colloidal nanoparticles. Orderly assembly of the perylene molecules along the inner walls of the pores of the AAO template most probably contributed to the Raman scattering as well as strengthened the interactions between Ag and perylene. The high-density ordered arrays of nano-pillars have resulted in a surface plasmon resonance, which gave rise to the large enhancement in the Raman intensity. This kind of physically assembled core–shell one-dimensional system may be an ideal SERS substrate for fluorescing molecules whose Raman spectra cannot be easily obtained.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 50221201, 90301010, 20471062, 50573084), and the Chinese Academy of Sciences.References
- J. Visser and E. J. J. Groenen, Chem. Phys. Lett., 2002, 356, 43–48 CrossRef CAS.
- S. J. Lee and K. Kim, Chem. Phys. Lett., 2003, 378, 122–127 CrossRef CAS.
- E. J. Ayars and H. D. Hallen, Appl. Phys. Lett., 2000, 76, 3911–3913 CrossRef CAS.
- A. Otto and A. Bruckbauer, J. Mol. Struct., 2003, 661–662, 501–514 CrossRef CAS.
- B. H. Loo, Y. Tse, K. Parsons, C. Adelman, A. El-Hage and Y. G. Lee, J. Raman Spectrosc., 2006, 137, 299–304 CrossRef.
- B. Pettinger, B. Ren, G. Picardi, R. Schuster and G. Ertl, Phys. Rev. Lett., 2004, 92, 096101 CrossRef.
- S. Nie and S. R. Emory, Science, 1997, 275, 1102 CrossRef CAS.
- K. Kneipp, Y. Wang, H. Kneipp, L. T. Perelman, I. Itzkan, R. R. Dasari and M. S. Feld, Phys. Rev. Lett., 1997, 78, 1667 CrossRef CAS.
- X. Xu, E. J. Bjerneld, M. Käll and L. Börjesson, Phys. Rev. Lett., 1999, 83, 4357–4360 CrossRef CAS.
- L. Zhao, W. Yang, Y. Ma, J. Yao, Y. Li and H. Liu, Chem. Commun., 2003, 2392–2393 RSC.
- F. X. Redl, K. S. Cho, C. B. Murray and S. O'Brien, Nature, 2003, 423, 968–971 CrossRef CAS.
- D. H. Son, S. M. Hughes, Y. D. Yin and A. P. Alivisatos, Science, 2004, 306, 1009–1012 CrossRef CAS.
- T. C. Harman, P. J. Taylor, M. P. Walsh and B. E. LaForge, Science, 2002, 297, 2229 CrossRef CAS.
- R. Venkatasubramanian, E. Siivola, T. Colpitts and B. O'Quinn, Nature, 2001, 413, 597 CrossRef CAS.
- A. L. Prieto, M. Martın-Gonzalez, J. Keyani, R. Gronsky, T. Sands and A. M. Stacy, J. Am. Chem. Soc., 2003, 125, 2388–2389 CrossRef CAS.
- H. Masuda and K. Fukuda, Science, 1995, 268, 1466 CrossRef CAS.
- H. Masuda, H. Yamada, M. Satoh, H. Asoh, M. Nakao and T. Tamamura, Appl. Phys. Lett., 1997, 71(19), 2770 CrossRef CAS.
- K. Nielsch, F. Müller, A. P. Li and U. Gösele, Adv. Mater., 2000, 12(8), 582 CrossRef CAS.
- Y. Cui and C. M. Lieber, Science, 2001, 291, 851 CrossRef CAS.
- A. L. Prieto, M. M. Gonzalez, J. Keyani, R. Gronsky, T. Sands and A. M. Stacy, J. Am. Chem. Soc., 2003, 125, 2388 CrossRef CAS.
- D. Xu, Y. Xu, D. Chen, G. Guo, L. Gui and Y. Tang, Chem. Phys. Lett., 2000, 325, 340 CrossRef CAS.
- A. P. Li, F. Müller, A. Birner, K. Nielsch and U. Gösele, Adv. Mater., 1999, 11(6), 483 CrossRef CAS.
- H. Masuda, H. Asoh and M. Watanabe, Adv. Mater., 2001, 13, 189–191 CrossRef CAS.
- T. M. Whitney, J. S. Jiang, P. C. Searson and C. L. Chien, Science, 1993, 261, 1316 CAS.
- R. J. Tonucci, B. L. Justus, A. J. Campillo and C. E. Ford, Science, 1992, 258, 783 CAS.
- C. G. Granqvist, A. Andersson and O. Hundri, Appl. Phys. Lett., 1979, 35, 268 CrossRef CAS.
- C. A. Huber, T. E. Huber, M. Sadoqi, J. A. Lubin, S. Manalis and C. B. Prater, Science, 1994, 263, 800.
- C. R. Martin, Science, 1994, 266, 1961 CrossRef CAS.
- Z. Luo and Y. Fang, Vib. Spectrosc., 2006, 41, 37 CrossRef CAS.
- S. Zhu, Z. Zhijie and L. Zhongze, Sci. Technol. Rev., 1999, 12 Search PubMed.
- L. Zhang, H. Hu, X. Wang and J. Liang, J. Cent. South Univ. Technol., Nat. Sci., 2003, 34, 510–512 Search PubMed.
- H. Zeng, H. Hu, J. Wei, F. Xie and P. Peng, Acta Phys. Sin., 2006, 09 Search PubMed.
- C. K. Preston and M. Moskovits, J. Phys. Chem., 1993, 97, 8495 CrossRef CAS.
- X. Jin, J. Y. Hu, Y. Wan, W. He and H. Han, China Water Wastewater, 2005, 21, 7 Search PubMed.
- D. L. Gerrard and W. F. Maddams, Appl. Spectrosc., 1976, 30(5), 554 CrossRef CAS.
- R. M. Hochstrasser and C. A. Nyi, J. Chem. Phys., 1980, 72(4), 2591–2600 CrossRef CAS.
- M. Moskovits, Rev. Mod. Phys., 1985, 57, 783–823 CrossRef CAS.
- P. C. Lee and D. Meisel, J. Phys. Chem., 1982, 86, 3391 CrossRef CAS.
- K. K. Ong, J. O. Jensen and H. F. Hameka, J. Mol. Struct., 1999, 459, 131.
- S. Akimoto, A. Ohmori and I. Yamazaki, J. Phys. Chem. B, 1997, 101, 3753–3758 CrossRef CAS.
- T. J. Kosic, C. L. Schosser and D. D. Dlott, Chem. Phys. Lett., 1983, 96, 57–64 CrossRef CAS.
- J. I. Gersten and A. Nitzan, J. Chem. Phys., 1980, 73, 3023 CrossRef CAS.
- K. Wang and Y.-S. Li, Vib. Spectrosc., 1997, 14, 183–188 CrossRef CAS.
- F. Lü, H. Zheng and Y. Fang, Prog. Chem., 2007, 19(2), 3 Search PubMed.
- A. Campion, A. R. Gallo, C. B. Harris, H. J. Robota and P. M. Whitmore, Chem. Phys. Lett., 1980, 73(397), 450.
- J. I. Gersten, J. Chem. Phys., 1980, 72, 5779 CrossRef CAS.
- P. F. Liao and A. Wokaun, Chem. Phys. Lett., 1982, 86(4), 397–400 CrossRef.
- Z.-q. Tian, B. Ren and D.-Y. Wu, J. Phys. Chem. B, 2002, 106, 37.
- B. Ren, G. Picardi and B/ Pettinger, Rev. Sci. Instrum., 2004, 75, 837–841 CrossRef CAS.
- M. Kasha, H. R. Rawls and M. A. El-Bayoumi, Pure Appl. Chem., 1965, 11, 371 CrossRef CAS.
- E. G. McRae and M. Kasha, J. Chem. Phys., 1958, 27, 721 CrossRef CAS.
- T. Katoh, Y. Inagaki and R. Okazaki, J. Am. Chem. Soc., 1998, 120, 3623 CrossRef CAS.
- E. S. Emerson, M. A. Conlin, A. E. Rosenoff, K. S. Norland, H. Rodriguez, D. Chin and G. R. Bird, J. Phys. Chem., 1967, 71, 2396 CrossRef CAS.
- H. J. Wu, Y. Fang and P. X. Zhang, J. Phys. Chem. B, 2005, 109(46), 21865–21867 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2008 |
