Carbon nanotube cell translocation and delivery of nucleic acidsin vitro and in vivo
Lara
Lacerda
a,
Alberto
Bianco
b,
Maurizio
Prato
c and
Kostas
Kostarelos
*a
aNanomedicine Laboratory, Centre for Drug Delivery Research, The School of Pharmacy, University of London, 29–39 Brunswick Square, London, UK, WC1N 1AX. E-mail: kostas.kostarelos@pharmacy.ac.uk; Tel: +44-0207-753 5861
bCNRS, Institut de Biologie Moléculaire et Cellulaire, Laboratoire d'Immunologie et Chimie Thérapeutiques, 67000 Strasbourg, France
cDipartimento di Scienze Farmaceutiche, Università di Trieste, 34127 Trieste, Italy
First published on 10th October 2007
Abstract
In the last few years, the carbon nanotube (CNT) field has seen a new direction of investigation growing rapidly, along with the interest of more researchers from diverse fields of expertise interested in this new material in an attempt to exploit their properties in biomedical applications. Here we describe the most recently reported work on the application of CNT for gene encoding nucleic acid (DNA and RNA) delivery purposes by using in vitro and in vivo models. Several groups have now successfully observed the cellular internalisation of nucleic acids with the aid of CNT following very different protocols. The main processes for the internalisation pathways and intracellular release of the nucleic acids are here reviewed. Furthermore, we have just started to see some initial studies of in vivo work using siRNA-CNT conjugates to achieve silencing in tumour tissue. Admittedly, it is still very early days for the technology, but future studies are necessary, and will surely appear, in order to determine the feasibility of bringing the CNT closer to the clinic.
 Lara Lacerda | Ms Lara Lacerda completed her First Degree in Pharmaceutical Sciences at the University of Coimbra in 2003. Currently she is pursuing a PhD degree in carbon nanotubes and nanomedicine at the University of London under the supervision of Kostas Kostarelos. Her research focuses on the development of functionalised carbon nanotubes for biomedical applications such as drug and gene delivery. Lara is an associate member of the Royal Society of Chemistry (UK). |
 Alberto Bianco | Alberto Bianco received his Laurea degree in Chemistry in 1992 and his PhD in 1995 from the University of Padova (Italy), under the supervision of Professor C. Toniolo. As a visiting scientist, he worked at the University of Lausanne during 1992 (with Professor M. Mutter), at the University of Tübingen in 1996–1997 (with Professor G. Jung, as an Alexander von Humboldt fellow) and at the University of Padova in 1997–1998 (with Professor G. Scorrano). He currently is a Research Director at CNRS in Strasbourg (France). His research interests focus on the development of carbon-based nanomaterials as therapeutic vectors; the applications of functionalized carbon nanotubes and fullerenes in nanomedicine; and the synthesis of fullero-peptides as new ligands for immunotherapy. He is member of the American Chemical Society, the French Group of Peptides and Proteins, and the European Peptide Society. |
 Maurizio Prato | Professor Maurizio Prato obtained his Laurea degree in chemistry in 1978 from the University of Padova, Italy, where he was appointed Assistant Professor in 1983. He moved to Trieste as an Associate Professor in 1992 and was promoted to Full Professor in 2000. He spent a postdoctoral year in 1986–1987 at Yale University, was a Visiting Scientist at the University of California, Santa Barbara, in 1991–92, and Professeur Invité at the Ecole Normale Supérieure in Paris, France, in July 2002. His research interests focus on the synthesis of novel functional materials, including functionalized fullerenes and carbon nanotubes, for applications in materials science and medicinal chemistry. His scientific contributions have been recognized by National awards, which include: Federchimica Prize (1995, Association of Italian Industries) and the National Prize for Research (2002, Italian Chemical Society). |
 Kostas Kostarelos | Professor Kostarelos is the Chair of Nanomedicine at The School of Pharmacy of the University of London, a Fellow of the Royal Society of Medicine (FRSM) and a Fellow of the Institute of Nanotechnology (FIoN). He obtained his Diploma in Chemical Engineering and PhD from the Department of Chemical Engineering, Imperial College London. Following his promotion to Assistant Professor of Genetic Medicine and Chemical Engineering in Medicine at Cornell University Weill Medical College, he relocated to the UK as the Deputy Director of Imperial College Genetic Therapies Centre. Prof. Kostarelos is the Senior Editor of the journal Nanomedicine and sits on the Editorial Board of: The Journal of Liposome Research, The International Journal of Nanomedicine, and is an International Editor for Nanomedicine: Nanotechnology, Biology and Medicine. |
1. Introduction
Carbon nanotubes (CNT) consist exclusively of carbon atoms arranged in a series of graphene-like planar sheets rings rolled up into a tubular structure. Generally, CNT are classified in two categories based on their structure and dimensions: single-walled carbon nanotubes (SWNT), which consist of one layer of cylindrical graphene and have diameters from 0.4 to 2.0 nm and lengths in the range of 20–1000 nm; and multi-walled carbon nanotubes (MWNT), which contain several concentric graphene sheets and greater dimensions with diameters in the range of 1.4–100 nm and lengths from 1 to a few microns. Several methods for the production of both types of tubes and the modulation of their dimensions have been described.1 CNT have attracted the attention of various research groups as the possible applications of these tiny carbon structures spread rapidly among very different fields. Their extraordinary properties, such as high electrical and thermal conductivity, great strength and rigidity, have been developed for a wealth of applications, including resilient composite materials,2 field emission,3 energy storage,4 and molecular electronics,5 among many others.Pristine (as-prepared) CNT aggregate readily in bundles and are difficult to suspend effectively as a consequence of strong van der Waals forces. Therefore, the application of CNT as novel ‘dry’ materials has been explored previously due to their lack of solubility in most solvents that posed an obstacle for the utilization of CNT in ‘wet’ applications such as in biology and medicine. However, after the discovery of functionalisation of CNT using different types of organic reactions it has been possible to individualise and disperse them in solvents. Common techniques used to functionalise CNT are: (a) defect-site, (b) covalent sidewall, (c) non-covalent exohedral and (d) endohedral functionalisation.6 The simplest method to achieve aqueous suspensions of CNT is by wrapping and coating using polymers, surfactants or biomolecules with the aid of sonication.6 An alternative approach is the introduction of chemical moieties on the nanotube surface via an organic reaction (covalent functionalisation), such as the 1,3-dipolar cycloaddition reaction, which has been primarily used in our laboratories.7 Once the issue of CNT aqueous dispersibility was dramatically improved, several research groups began investigating the potential of CNT in the fields of biotechnology and biomedicine,8,9 since the development of water-soluble CNT automatically renders them usable in contact with biological fluids and environments.
A further important development was the discovery that CNT can non-covalently bind single stranded10 and double stranded11,12DNA. Due to the latter, the complex formation between CNT and nucleic acids allowed the exploration of the hypothesis that CNT can be used as gene delivery systems. In the present article we highlight the current state-of-the-art in the delivery of nucleic acids by using CNT that have used both in vitro and in vivo models, and highlight the development of CNT as gene delivery vectors.
2. Delivery of nucleic acids by CNT
Exogenous gene delivery and expression in live cells is a complex biological phenomenon that has been applied for a variety of purposes, most commonly gene identification and gene therapy. The optimum delivery of genes in diseased tissue is considered today to be a bottleneck in both viral (i.e. use of genetically modified viruses) and non-viral (i.e. use of chemical moieties) gene therapy applications. There is an ever-growing need to enhance the available tools that can lead to effective and safe gene delivery and expression. CNT are some of the most recent nanomaterial constructs to be developed as gene delivery vectors, stemming from both their attractive physicochemical and electronic features as well as the initial observations that they can be internalised in mammalian cells very efficiently.13,14 A summary of the different studies published today using CNT for the delivery of nucleic acids in in vitro and in vivo models is presented in Table 1.| Nucleic acid | CNT | Model | Ref. | |
|---|---|---|---|---|
| In vitro | ||||
| DNA | pDNA (βgal) | SWNT-NH3+ | CHO cells | 11 |
| pDNA (βgal) | SWNT-NH3+ | A459 cells | 12 | |
| SWNT-Lys-NH3+ | ||||
| MWNT-NH3+ | ||||
| pDNA (CMV-Luc) | MWNT | 293 cells | 15 | |
| MWNT-PEI | COS7 cells | |||
| MWNT-ox-NH2 | HepG2 cells | |||
| MWNT-ox-NH-PEI | ||||
| MWNT-ox-NH-PEI-FITC | ||||
| pDNA (EGFPN1) | MWNT-NH2 | HUVEC cells | 16 | |
| MWNT-COOH | A375 cells | |||
| MWNT-OH | ||||
| MWNT-CH2CH2CH3 | ||||
| pDNA (EGFP-c1) | CNT(Ni)-COOH | Bal17 cells | 17 | |
| CNT-COOH | mouse splenic B cells and primary culture of mice cortical neurons | |||
| pDNA (UC19) | MWNT-COOH | E. coli strain DH5α | 18 | |
| FAM-dsDNA-NH2 | SWNT-COOH | 1H8 cells | 20 | |
| TC-1 cells | ||||
| LLC cells | ||||
| Cy3-ssDNA | SWNT | HeLa cells | 22,23 | |
| HL60 cells | ||||
| Cy3-ssDNA-SH | SWNT-DGPE-PEG-SH and | HeLa cells | 24 | |
| SWNT-DGPE-PEG-maleimide | ||||
| ssDNA | SWNT | Murine myoblast stem cells | 25,26 | |
| 3T3 cells | ||||
| Cy3-ssDNA | SWNT (long and short) | IMR 90 cells | 27 | |
| Cy5-ssDNA | A549 cells | |||
| MC3T3-E1 cells | ||||
| A10 cells | ||||
| RNA | PI-poly(rU) RNA | SWNT | MCF7 cells | 28 |
| siRNA-SH | SWNT-DGPE-PEG-SH and | HeLa cells | 24 | |
| SWNT-DGPE-PEG-maleimide | ||||
| siRNA (murineTERT) | SWNT-CONH-(CH2)6NH3+ | 1H8 cells | 20 | |
| siRNA (humanTERT) | TC-1 cells | |||
| LLC cells | ||||
| HeLa cells | ||||
| SH-siRNA (CXCR4) | SWNT-PL-PEG2000-SH | Human T cells and | 29 | |
| SH-siRNA (CD4) | SWNT-PL-PEG5400-SH | peripheral blood mononuclear cells | ||
| SH-siRNA (Luc) | ||||
| In vivo | ||||
| DNA | FAM-dsDNA-NH2 | SWNT-COOH | Tumour-bearing mice | 20 |
| RNA | si-RNA (murineTERT) | SWNT-CONH-(CH2)6NH3+ | Tumour-bearing mice | 20 |
| si-RNA (humanTERT) | ||||
2.1 In vitro models
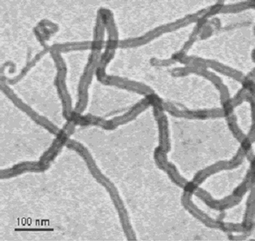 | ||
| Fig. 1 Water soluble MWNT functionalised through the organic 1,3-dipolar cycloaddition reaction. | ||
The next study to reproduce the concept was that by Liu et al.15 who reported the non-covalent association of pDNA with CNT functionalised with polyethyleneimine (PEI) groups, which contain a high density of terminal amine groups, an overall highly cationic macromolecule commonly used as a transfection agent. The CNT-PEI/pDNA complexes tested at different charge ratios in different cell lines were shown to produce much higher levels of gene expression than pDNA alone. Furthermore, it was reported that the transfection efficiency with the CNT–PEI constructs was about 3 times higher than with PEI alone, postulating a similar endosome-mediated intracellular uptake. However, the exact mechanism leading to the reported upregulation of gene expression by the CNT–PEI conjugate was not identified.
Later, work reported by Gao et al.16 elucidated that the surface functionalisation group of the CNT also plays a role in the formation of the complexes between the CNT and pDNA. That team studied the gene delivery of pDNA by CNT functionalised with four different chemical groups: amino, carboxyl, hydroxyl and alkyl. They found that only the positively charged, amine-functionalised CNT were able to complex and deliver the pDNA. Similar to our own original observations, this report described that complexes between CNT and pDNA enhanced the gene delivery and expression compared to naked DNA alone, however they did not improve the transfection efficiency compared to a commercially available transfection agent (Lipofectamine 2000).
Cai et al.17 proposed a new strategy to introduce exogenous pDNA immobilised on CNT into mammalian cells. The CNT used in these studies contained Ni particles enclosed in their tips, which allowed a magnetic ‘spearing’ technique (exposure to an external magnetic field followed by centrifugation) onto the CNT–pDNA complexes. The results from these investigations reported gene expression in 80–100% of the cell population for the CNT with Ni, while CNT deprived of Ni particles did not produce any gene expression. Although, the use of such technique is limited to in vitro or ex vivogene transfer, this was the first work to utilise the plasma membrane ‘piercing’ effect that we originally proposed in an alternative experimental setup and that can lead to very high gene expression.
Interestingly, CNT have been shown to be able to deliver exogenous genes not only into mammalian cells but also into bacteria. Rojas-Chapana et al.18 demonstrated that oxidised, water-dispersible CNT can deliver pDNA into E. coli (ratio of transformation efficiency/transformants of about 32) by opening up temporary nanochannels across the cell envelope. The authors described that addition of CNT in a suspension containing E. coli and pDNA and application of a microwave frequency19 resulted in the orientation of the CNT tips perpendicularly to the cell surface and subsequently plasmid delivery into the bacteria. In the authors' own words “CNT appear to be needle-pricking the cells”. Again the concept of cell wall ‘piercing’ was proposed to explain the observations made in these studies. Zhang et al.20 have confirmed that CNT functionalised with short dsDNA are internalized by different murine tumour cell lines without any associated cytotoxic effects. The conjugates were tracked by fluorescence microscopy, since the dsDNA was fluorescently labeled. This work did not describe gene expression using this dsDNA. Moreover, very recently our original observation and subsequent hypothesis of CNT acting as ‘nanoneedles’ of the plasma membrane has been almost identically reproduced using CNT non-covalently functionalised with block copolymer molecules interacting with microglia cells.21 The work accumulating gradually by different groups is confirming our proposals that novel, very interesting mechanisms other than endocytosis are contributing to the high levels of cellular internalisation of CNT.
There have also been a few published studies using single-stranded DNA (ssDNA) as the CNT coating material to render the CNT water dispersible. Of course such nucleic acids do not encode for exogenous genes, therefore are not relevant to gene delivery applications, however intracellular translocation of the ssDNA is reported. Kam et al. have reported the translocation of ssDNA into the nuclei of HeLa cells by two different CNT structures: (a) Cy3-labeled ssDNA directly linked to CNT through a non-covalent adsorption22,23 and (b) Cy3-labeled ssDNA covalently linked to a polyethylene glycol chain that was part of a phospholipid molecule which was adsorbed onto the CNT.24 These studies reported that ssDNA is released from the CNT after NIR excitation of the CNT23 or after enzymatic cleavage of the disulfide bonds24 and translocated into the cell nucleus. The authors postulate endocytotic uptake of the whole CNT–ssDNA constructs. Heller et al.25,26 have also reported the cellular internalisation of CNT coated by ssDNA. Although the long-term goal of this team is to develop resilient optical sensors based on CNT for in vitro and in vivo applications, they have used ssDNA to achieve the solubilisation of the CNT in aqueous solutions and demonstrated an endocytosis-dependent internalisation and perinuclear localisation without penetration into the nuclear envelope of the CNT–ssDNA complexes incubated with mammalian cells.
More recently, Becker et al.27 have reported the importance of length on the corresponding toxicity of CNT–ssDNA complexes that are uptaken into cells (approx. 200 nm). This report showed length-dependent cellular uptake by fluorescence microscopy. Longer CNT were labelled with Cy3-ssDNA and found to be excluded from internalisation by cells, while shorter tubes labelled with Cy5-ssDNA were found in the cytoplasm. Although this study was oriented towards the length-dependent toxic effects of CNT, one can conclude that CNT–ssDNA complexes are able to internalise into a variety of cell lines. What should be highlighted however from these studies using CNT dispersions coated with macromolecules is that the surface coating on the CNT, ssDNA or PEGylated lipid in the cases above, is—as expected—critically important in the interactions between cells and CNT. The endocytotic mechanism of uptake reported is thought to be a result of the nucleic acid or lipid coat on the CNT surface recognised by the cells instead of the CNT backbone.
In a subsequent publication, Kam et al.24 have studied a siRNA–CNT conjugate linked with disulfide bonds to PEGylated lipids coating the CNT surface, and have shown that the conjugates were internalised into HeLa cells and also reported siRNA-mediated gene silencing. In these studies, different siRNA constructs were successfully delivered by CNT, and two targeted genes were silenced: (a) the gene encoding lamin A/C protein (localised inside the nuclear lamina of cells) and (b) the luciferase gene. This work also compared the gene silencing efficiency between the siRNA–CNT conjugates and the more conventional Lipofectamine–siRNA complexes to find that the CNT improved the degree of gene silencing.
The work reported by Zhang et al.20 pushed the development of CNT as siRNA delivery systems further, as they employed ammonium-functionalised CNT to mediate the delivery of TERTsiRNA into tumour cells (murine and human) and silence the TERT gene, which is critical for the development and growth of tumours. They observed that treatment of different tumour cells with the CNT–TERTsiRNA complexes led to the suppression of the cell growth. More recently, and with an alternative therapeutic endpoint, Liu et al.29 have shown the delivery of siRNA molecules able to silence the expression of the cell-surface receptors CD4 and co-receptors CXCR4 in human T cells and peripheral blood mononuclear cells by CNT. Because both cell-surface receptors are necessary for HIV access and infection of T cells, the authors have suggested that siRNA–CNT conjugates can potentially be used for the treatment of HIV. Furthermore, they observed that conjugates of CNT covalently linked by disulfide bonds to siRNA had greatly improved the silencing in T cells compared to Lipofectamine 2000 and other liposome-based formulations.
Fig. 2 schematically summarizes the general strategies that have been described to introduce nucleic acid–CNT conjugates into cells, regardless of the structure of the nucleic acid and the achievement of gene expression (DNA) or protein silencing (siRNA). The main internalisation pathways to accomplish cellular delivery of nucleic acids by CNT are as follows (Fig. 2): phagocytosis of nucleic acids covalently linked to CNT29 (A), injection of nucleic acids through nanochannels formed by CNT18 (B), ‘penetration’ or ‘piercing’ of nucleic acids adsorbed on the surface of CNT17 (CI) or complexed with CNT by electrostatic forces11,12 (CII); and finally, endocytosis of nucleic acids electrostatically complexed15 (DI), covalently linked24 (DII) or adsorbed22,23,25 (DIII) to CNT. The mechanisms suggested to describe intracellular release of the nucleic acid from the CNT are illustrated as well in Fig. 2 as follows: electrostatic dissociation of the complexes11,12,15,16 (E); enzymatic cleavage of the disulfide linkages that hold the nucleic acid onto the CNT24 (F); and lastly, nucleic acid release following excitation of the CNT with NIR radiation23 (G).
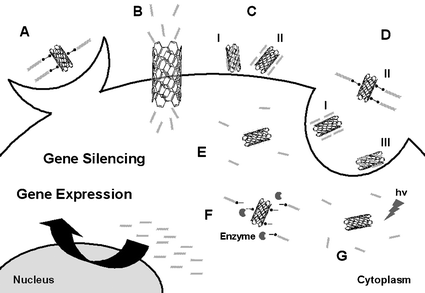 | ||
| Fig. 2 Suggested strategies for cellular delivery of nucleic acids by CNT: phagocytosis of nucleic acids covalently linked to CNT29 (A), injection of nucleic acids through CNT nanochannels18 (B), penetration of nucleic acids adsorbed on the surface of CNT17 (CI) or complexed with CNT by electrostatic forces11,12 (CII); and endocytosis of nucleic acids electrostatically complexed15 (DI), covalently linked24 (DII) or adsorbed22,23,25 (DIII) to CNT. Suggested strategies for intracellular release of the nucleic acid from the CNT: electrostatic dissociation11,12,15,16 (E), enzymatic cleavage24,29 (F) and radiation23 (G). | ||
2.2 In vivo models
3. Future work and directions
Several biomedical applications of CNT have been proposed in the last few years. Concerning the utilisation of CNT in the delivery of nucleic acids, the encouraging findings described so far in the literature are highlighted herein. CNT are versatile platforms for nucleic acid delivery in vitro and in vivo because of their high surface area, facile functionalisation of their surface, and their ability to cross the cell membranes. To our knowledge, it is crucial to functionalise the surface of CNT, in order to transform non-functionalised CNT (insoluble in most solvents) into water-soluble and biocompatible CNT with an improved toxicity profile. On the other hand, with a growing number of functionalisation routes, many important questions remain unanswered. Each functionalisation method is probably producing CNT with different characteristics, which will lead to differences in the mechanism of CNT metabolism, degradation or dissolution, clearance and bioaccumulation. On the other hand, most non-viral gene delivery systems today suffer from both limited levels of gene expression and an unfavorable toxicity profile due to their highly cationic surface character. Therefore, opportunities for CNT-based gene transfer systems are still ample.The development of CNT as delivery systems for nucleic acids is still in its nascent stages and many more studies are needed to determine the advantages and limitations offered by these novel materials. Most challenges are still ahead for the biomedical utilisation of CNT. The administration or implantation of CNT and their complexes with gene encoding or silencing constructs in an in vivo setting are almost completely unexplored, despite the groundbreaking work by several groups.30 Moreover, the toxicological and pharmacological profiles of CNT systems used as therapeutics will have to be better elucidated prior to any clinical studies undertaken. In conclusion, we would like to stress that although we are still in very early stages of development, nucleic acid delivery systems based on CNT may well become viable and effective, particularly in combination with their electrical or semiconducting properties and an improved toxicity profile.
Acknowledgements
Our gratitude goes to all co-workers who have contributed to the development of the research partly described in this article and whose names are cited in the references. This work has been supported by the CNRS, the “Agence Nationale de la Recherche” (grant ANR-05-JCJC-0031-01), the University of Trieste, MUR (PRIN 2006, prot. 2006034372 and Firb RBIN04HC3S) and Regione Friuli Venezia-Giulia (art. 11 L. R. 11/2003) and the European Union, NEURONANO program (NMP4-CT-2006-031847). L. Lacerda is grateful to the Portuguese Foundation for Science and Technology (FCT/MCES) for a PhD fellowship (Ref.: SFRH/BD/21845/2005).References
- Special Issue on Carbon Nanotubes: Acc. Chem. Res., 2002, 35, 997 Search PubMed.
- M. Moniruzzaman and K. I. Winey, Macromolecules, 2006, 39, 5194 CrossRef CAS.
- M. Terrones, Int. Mater. Rev., 2004, 49, 325 Search PubMed.
- A. S. Arico, P. Bruce, B. Scrosati, J. M. Tarascon and W. Van Schalkwijk, Nat. Mater., 2005, 4, 366 CrossRef CAS.
- A. P. Graham, G. S. Duesberg, R. V. Seidel, M. Liebau, E. Unger, W. Pamler, F. Kreupl and W. Hoenlein, Small, 2005, 1, 382 CrossRef CAS.
- D. Tasis, N. Tagmatarchis, A. Bianco and M. Prato, Chem. Rev., 2006, 106, 1105 CrossRef CAS.
- V. Georgakilas, K. Kordatos, M. Prato, D. M. Guldi, M. Holzinger and A. Hirsch, J. Am. Chem. Soc., 2002, 124, 760 CrossRef CAS.
- Y. Lin, S. Taylor, H. P. Li, K. A. S. Fernando, L. W. Qu, W. Wang, L. R. Gu, B. Zhou and Y. P. Sun, J. Mater. Chem., 2004, 14, 527 RSC.
- K. Kostarelos, L. Lacerda, C. D. Partidos, M. Prato and A. Bianco, J. Drug Delivery Sci. Technol., 2005, 15, 41 Search PubMed.
- M. Zheng, A. Jagota, E. D. Semke, B. A. Diner, R. S. McLean, S. R. Lustig, R. E. Richardson and N. G. Tassi, Nat. Mater., 2003, 2, 338 CrossRef CAS.
- D. Pantarotto, R. Singh, D. McCarthy, M. Erhardt, J. P. Briand, M. Prato, K. Kostarelos and A. Bianco, Angew. Chem., Int. Ed., 2004, 43, 5242 CrossRef CAS.
- R. Singh, D. Pantarotto, D. McCarthy, O. Chaloin, J. Hoebeke, C. D. Partidos, J. P. Briand, M. Prato, A. Bianco and K. Kostarelos, J. Am. Chem. Soc., 2005, 127, 4388 CrossRef CAS.
- D. Pantarotto, J. P. Briand, M. Prato and A. Bianco, Chem. Commun., 2004, 16 RSC.
- L. Lacerda, G. Pastorin, D. Gathercole, J. Buddle, M. Prato, A. Bianco and K. Kostarelos, Adv. Mater., 2007, 19, 1480 CrossRef CAS.
- Y. Liu, D. C. Wu, W. D. Zhang, X. Jiang, C. B. He, T. S. Chung, S. H. Goh and K. W. Leong, Angew. Chem., Int. Ed., 2005, 44, 4782 CrossRef CAS.
- L. Gao, L. Nie, T. Wang, Y. Qin, Z. Guo, D. Yang and X. Yan, ChemBioChem, 2006, 7, 239 CrossRef CAS.
- D. Cai, J. M. Mataraza, Z. H. Qin, Z. Huang, J. Huang, T. C. Chiles, D. Carnahan, K. Kempa and Z. Ren, Nat. Methods, 2005, 2, 449 CrossRef CAS.
- J. Rojas-Chapana, J. Troszczynska, I. Firkowska, C. Morsczeck and M. Giersig, Lab Chip, 2005, 5, 536 RSC.
- J. A. Rojas-Chapana, M. A. Correa-Duarte, Z. F. Ren, K. Kempa and M. Giersig, Nano Lett., 2004, 4, 985 CrossRef CAS.
- Z. Zhang, X. Yang, Y. Zhang, B. Zeng, S. Wang, T. Zhu, R. B. Roden, Y. Chen and R. Yang, Clin. Cancer Res., 2006, 12, 4933 CrossRef CAS.
- B. Kateb, M. V. Handel, L. Zhang, M. J. Bronikowski, H. Manohara and B. Badie, NeuroImage, 2007, 37, S9 CrossRef.
- N. W. S. Kam, Z. A. Liu and H. J. Dai, Angew. Chem., Int. Ed., 2006, 45, 577 CrossRef.
- N. W. Kam, M. O'Connell, J. A. Wisdom and H. Dai, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 11600 CrossRef CAS.
- N. W. Kam, Z. Liu and H. Dai, J. Am. Chem. Soc., 2005, 127, 12492 CrossRef CAS.
- D. A. Heller, S. Baik, T. E. Eurell and M. S. Strano, Adv. Mater., 2005, 17, 2793 CrossRef CAS.
- D. A. Heller, E. S. Jeng, T. K. Yeung, B. M. Martinez, A. E. Moll, J. B. Gastala and M. S. Strano, Science, 2006, 311, 508 CrossRef CAS.
- M. L. Becker, J. A. Fagan, N. D. Gallant, B. J. Bauer, V. Bajpai, E. K. Hobbie, S. H. Lacerda, K. B. Migler and J. P. Jakupciak, Adv. Mater., 2007, 19, 939 CrossRef CAS.
- Q. Lu, J. M. Moore, G. Huang, A. S. Mount, A. M. Rao, L. L. Larcom and P. C. Ke, Nano Lett., 2004, 4, 2473 CrossRef CAS.
- Z. Liu, M. Winters, M. Holodniy and H. Dai, Angew. Chem., Int. Ed., 2007, 46, 2023 CrossRef CAS.
- L. Lacerda, A. Bianco, M. Prato and K. Kostarelos, Adv. Drug Delivery Rev., 2006, 58, 1460 CrossRef CAS.
Footnote |
| † The paper by Yang et al., Single-walled carbon nanotubes-mediated in vivo and in vitro delivery of siRNA into antigen-presenting cells, published online on 6 July 2006 in Gene Therapy, was recently retracted in Gene Therapy, 2007, 14, 920. doi:10.1038/sj.gt.3302962. |
| This journal is © The Royal Society of Chemistry 2008 |
