Highly sensitive pneumatic nebulization flame furnace atomic absorption spectrometry: complete sample aerosol introduction and on-line preconcentration of cadmium by atom trap
Peng
Wu
a,
Rui
Liu
b,
Harald
Berndt
c,
Yi
Lv
b and
Xiandeng
Hou
*ab
aAnalytical & Testing Center, Sichuan University, Chengdu, Sichuan 610064, China. Fax: +86 28 8541 2907; E-mail: houxd@scu.edu.cn, houxiandeng@yahoo.com.cn
bCollege of Chemistry, Sichuan University, Chengdu, Sichuan 610064, China
cInstitute for Analytical Sciences, Bunsen-Kirchhoff-Str. 11, 44139 Dortmund, Germany
First published on 17th October 2007
Abstract
Complete introduction of sample aerosol into flame AAS is achieved by hyphenating pneumatic nebulization (PN) with flame furnace AAS (FF-AAS). The analyte solution is introduced via a pneumatic nebulizer into the flame heated furnace by a flow of carrier gas (air or argon). The middle part of the flame furnace, where the carrier gas impacts, is cooled by the gas flow as well as the sample aerosol, and this provides a fine strategy for on-line atom trapping for the purpose of preconcentration. A stainless steel plate is put on the top of the flame burner in the middle to form a flame-free zone, which also lowers the temperature of the flame furnace and facilitates the atom trapping process. Cadmium is selected as the analyte element because its low temperature atomization characteristic most suits for this approach. With 10 s preconcentration time (corresponding to 300 µL sample solution), a sensitivity enhancement factor of 730 (over conventional flame AAS) is obtained. The limit of detection for cadmium by the proposed method was found to be 15 ng L–1, comparable to that by graphite furnace AAS or chemical vapour generation atomic fluorescence spectrometry. This superior detectability originates from the atom trapping step involved in this simple and cost-effective approach.
1. Introduction
Flame atomic absorption spectrometry (FAAS) is one of the most widespread traditional analytical techniques for the determination of trace elements because of its robustness and relatively low cost, but it often suffers from low sensitivity in ultra-trace analysis because of the low nebulization efficiency and the short residence time of free atoms in the flame. Obviously, the detection power of FAAS can be improved by increasing the efficiency of aerosol generation/transport and prolonging the residence time of the free analyte atoms in the absorption volume. There have been many attempts to realize complete sample introduction, such as a total consumption nebulizer–burner and Delves-cup system.1 Also, efforts have been made to prolong the residence time of analyte atoms by means of “atom trap” techniques.2 In spite of all these efforts made in past decades, the sensitivity of FAAS is still not comparable to that of the more sophisticated instrument of its kind, graphite furnace AAS.Flame furnace atomic absorption spectrometry (FF-AAS) is a recent powerful FAAS model with two variants: beam injection FF-AAS (BIFF-AAS)3 and thermospray FF-AAS (TS-FF-AAS).4,5 FF-AAS involves both complete introduction of whole sample into the atomization cell and considerably extended residence time of analyte in the absorption volume, thus leading to a marked sensitivity improvement over conventional FAAS. Owing to its robustness, simplicity, high sensitivity and cost-effectiveness, FF-AAS plays a unique role in ultra-trace analysis of various elements in a variety of sample matrices.6–9 Because of the oxidative atmosphere inside the flame furnace, simultaneous sample digestion and determination inside the tube (flame furnace) can be easily achieved using slurry sampling.10–12 In particular, using FF-AAS as an on-line element-selective detector for capillary electrophoresis has also been a success.13
In either beam injection FF-AAS or thermospray FF-AAS, the sample introduced into flame furnace cannot touch the inner wall of the furnace due to Leidenfrost phenomenon.3,14 If too much liquid is introduced, the temperature inside the tube will decrease greatly, thus lowering the sensitivity of determination. On the other hand, in common atom/hydride trapping techniques, a “cold” surface is a prerequisite for trapping free atoms15 as well as hydride.16,17 From this point of view, the flame furnace can be used as an atom trapping tube given that the temperature of the flame furnace can be lowered effectively. Therefore, this paper describes a new strategy for on-line cold-trap preconcentration of cadmium using complete pneumatic nebulization (PN) flame furnace atomic absorption spectrometry. In this study, the PN-FF-AAS is first established and then evaluated for on-line preconcentration and determination of cadmium by atom trap, with a metal plate on top of the burner to partially cover the flame under the middle of the flame furnace to help lower the temperature in order to facilitate the atom trapping process. This is the first time that the dream of complete introduction of aerosol into flame AAS has become a reality. It is also a unique on-line preconcentration technique for sensitivity enhancement for flame atomic absorption spectrometry.
2. Experimental
2.1. Instrumentation
The instrumental system described in this paper consists of a flame furnace (nickel tube) atomic absorption spectrometer and a pneumatic nebulizer. The sample is introduced via a standard concentric ICP pneumatic nebulizer (Meinhard type) into the flame furnace; it is nebulized, atomized and trapped on the inner surface of the furnace. The carrier gas (air or argon) is introduced via the outer tube of the concentric nebulizer and meets the sample flow at the outlet of the nebulizer to form a spray cone. Owing to the special design, high pressure is formed at the outlet of the nebulizer and the sample flow is broken into small pieces by the carrier gas. When the sample aerosol sprays out, it sucks air from the surroundings. The sample aerosol plus the sucked air causes strong cooling to the inner surface of the flame furnace where the sample aerosol impacts. Introduced sample is firstly atomized and then trapped onto the cooled surface immediately. During this sampling and atom trapping stage, a stainless steel plate is put on the top of the flame burner to further cool down the flame furnace for the purpose of atom trapping (Fig. 1).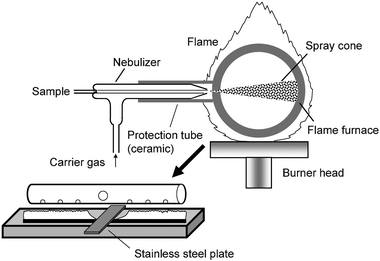 | ||
| Fig. 1 Instrumental arrangement of the PN-FF-AAS (not to scale). A stainless plate is put on the top of the burner to partially cover the flame and generate a flame-free zone which lowers the temperature of the flame furnace and facilitates the atom trapping process. During the sampling and atom trapping stage, the spray nozzle of the nebulizer is moved to the sampling hole of the nickel tube for sampling. | ||
The instrumental arrangement is shown in Fig. 1. A Model GGX-9 flame atomic absorption spectrometer equipped with D2 lamp background correction (Geological Instrument Co., Beijing, China) was used throughout this work. Working parameters (lamp current: 4 mA; spectral bandpass: 0.4 nm) were as recommended by the manufacturer. For all experiments in this work, 6 L min–1 air–1.5 mL min–1acetylene was used for the air–acetylene flame. A nickel tube of 10/12 mm id/od and 10 cm in length (J. & J. Ethen, Aachen, Germany) was used, with six holes of 2.5 mm diameter at the bottom to allow flame gases to enter the tube. The arrangement of the flame furnace was similar to that used in our previous work.18 A standard concentric ICP nebulizer (Meinhard type, Beijing Hanshi Manufacturing Factory, Beijing, China) was used in this work and was fixed onto a moveable holder, which allows the nebulizer system to move horizontally. In order to facilitate sample introduction, the initial sample introduction hole of the nickel tube was enlarged to 4 mm id. Because the nebulizer is made of glass, a ceramic tube (5 cm in length, 5 mm id) is mounted to protect the nebulizer. Furthermore, a piece of stainless steel plate (4 × 1 cm) is put on the burner head just below the nebulizer to generate a flame-free zone, which helps to lower the temperature of the flame furnace in order to facilitate the atom trapping process. When sampling finishes, the nebulizer is moved away and the temperature inside the flame furnace goes up immediately for atomization of the trapped cadmium to produce an atomic absorption signal. Sample delivering was aided by a peristaltic pump (Model HL-2, Huxi Analytical Instrumental Co., Shanghai, China).
2.2. Reagents
All reagents used were of the highest purity available but at least of analytical grade. Sub-boiled doubly distilled water (DDW) was used to prepare solutions throughout this work. Standard stock solutions of Cd, Pb, Hg, Zn, Tl and Ag (100 mg L–1) were purchased from the National Research Center for Standard Materials (NRCSM, Beijing, China). Working solutions in the µg L–1 and sub-µg L–1 range were obtained by stepwise dilution from the stock solution. Inorganic acids (HCl and HNO3) and salts (NaCl, KNO3, MgSO4, Ca(NO3)2) were purchased from Chengdu Kelong Chemical Reagents Co. (Chengdu, China).2.3. Samples and sample pretreatment
To validate the accuracy of the developed method, several certified reference materials were obtained from NRCSM: GBW 08607, GBW 08608 and GBW (E) 080401, Trace Elements in Water; GBW 08511 and GBW 08510, Cadmium in Rice; GBW 07605, Tea Leaf; and GBW 10014, Cabbage. Water samples were diluted directly and then subjected to analysis. Botanical samples were decomposed using microwave-assisted digestion based a previous method for corn digestion.192.4. Operating procedure
After arranging the instrumental setup, the nebulizer system is moved to connect the flame furnace. Then the peristaltic pump is activated and sample is delivered to the nebulizer. The sample aerosol is firstly atomized and then trapped on-line on the cold wall of the flame furnace. When the nebulizer gas is on, the cooling of the flame furnace can be visually observed. The cooling of the flame furnace is mainly ascribed to the nebulizer gas blast as the colour of the tube showed no significant difference with or without sampling. For a typical run, 10 s sampling time is allowed, and then the pump as well as the carrier gas is stopped. Meanwhile, the peak-height absorbance is recorded for quantification.3. Results and discussion
3.1. Complete introduction of sample aerosol into flame furnace AAS
In conventional FAAS or ICP spectrometry, in order to vaporize and atomize the sample aerosol as quickly and efficiently as possible, the diameter of the aerosol should be strictly controlled. Besides, the smaller size of aerosol further ensures the stability of the flame or plasma. Consequently, a spray chamber is indispensable for separating large liquid drops from the aerosol, and this is the reason for the low sample introduction efficiency of pneumatic nebulization in traditional atomic spectrometry. With the present approach, the dream of complete introduction of aerosol into flame AAS is realized, and this greatly increases the relative sensitivity for the measurement of cadmium either in terms of peak height or peak area measurement, compared with conventional FAAS or TS-FF-AAS, as can be seen from Fig. 2. With complete sample introduction, the instability of the PN-FF-AAS measurement is not significantly increased, compared with that by conventional FAAS or TS-FF-AAS, most probably owing to the atom trapping step which leads to delayed measurement after sampling. | ||
| Fig. 2 Comparison of recorded PN-FF-AAS signal with conventional FAAS and TS-FF-AAS. TS-FF-AAS working parameters were based on ref. 18. | ||
3.2. Selecting cadmium as the target analyte
In either BIFF-AAS or TS-FF-AAS, due to the fact that the temperature inside the flame furnace (about 1000 °C) is lower than the gas phase temperature of flame, the number of detectable elements is limited compared with conventional FAAS.3–5 Only those elements with a relatively low appearance temperature can gain superior sensitivity over conventional FAAS, for instance, Cd, Hg, Pb, Cu, Zn, Tl, and Ag. As the nebulizer causes a strong cooling effect to the flame furnace, it is expected that even fewer elements can be atomized in the present system. So elements with low appearance temperatures (Hg, Cd, Pb, Tl, Zn and Ag) were initially selected for this investigation. The results are summarized here. (1) No signal was obtained for Hg, even with a concentration as high as 10 µg mL–1, and further lowering of the flow rate of C2H2 (lowering the temperature) did not help. This was due to the extremely high volatility of mercury, which was volatilized before being trapped onto the nickel surface. (2) An obvious signal was observed with Cd. As compared with Hg, the appearance temperature of Cd is higher than that of Hg and thus it can be atomized and trapped onto the nickel surface. Fig. 2 shows the temporal signal profiles of Cd determined by PN-FF-AAS, conventional FAAS and TS-FF-AAS, respectively. It is clear that PN-FF-AAS shows a sensitivity superior to those conventional FAAS and TS-FF-AAS. (3) For Pb, Tl, Zn and Ag (elements with higher appearance temperatures than Cd20), after stopping the sample flow and carrier gas, no signals could be observed unless the stainless steel plate was pulled out from the flame (to further increase the atomization temperature). The signals appeared about 5–8 s after the plate was pulled out and they lasted about 1–1.5 min, with serious tailing. All these effects led to inferior analytical precision. When pulling out the plate, the cooled part of the tube was re-heated by the flame. Because the appearance temperatures of these elements are higher than that of Cd, the release of the atoms trapped on the inner wall of the nickel tube was much slower, leading to much broader signal peaks. Quantification of these elements can be difficult using either peak-height or peak-area absorbance due to the poor reproducibility. On the basis of the above observation, Cd was selected as the target analyte for this investigation.3.3. Optimization of instrumental parameters
 | ||
| Fig. 3 The influence of the plate width on the absorbance of 0.5 µg L–1 Cd (A) and tube wall temperature of the flame furnace (B). | ||
The effect of the carrier gas (Ar) flow rate on the signal of Cd is shown in Fig. 4. From Fig. 4 it can be seen that the absorbance of Cd increases dramatically with the Ar flow rate from 100 to 300 mL min–1 and then levels off. When the flow rate of the carrier gas is too low, the nebulization efficiency is poor and large liquid drops are obtained at the tip of the nebulizer. Owing to the Leidenfrost phenomenon, these liquid drops cannot get energy from the tube wall efficiently and thus cannot be atomized and trapped. An Ar flow rate of 300 mL min–1 is used for the following experiments.
 | ||
| Fig. 4 The effect of carrier gas flow rate on the absorbance of Cd. Experimental conditions: Cd, 0.5 µg L–1; plate width, 1 cm; sample volume, 300 µL. | ||
3.4. Interference evaluation
Interference studies were carried out in order to evaluate the selectivity of the proposed method. The influence of four macro constituents (K, Na, Ca and Mg, as their salts) on the determination of 0.5 µg L–1 Cd were investigated and the results were shown in Fig. 5. The concentrations of the interfering salts varied from 0.002 to 1% (m/V), equivalent to the concentration ratio of coexisting salt to analyte varying from 4 × 104 to 2 × 107. As shown, Fig. 5, KNO3 and Ca(NO3)2 cause no significant interference if their concentration is less than 0.1% (1000 µg mL–1) and 0.02% (200 µg mL–1), respectively, while the tolerable limits for NaCl and MgSO4 were both 0.005% (50 µg mL–1). Considering the melting points of these salts (listed in the caption for Fig. 5), we may conclude that the higher melting temperature of the salt, the more significant the interference on Cd determination. In the case of BIFF-AAS or TS-FF-AAS, the energy of the flame furnace is sufficient to vaporize salts and up to 10% NaCl causes no significant interference with copper determination.3,10 However, in the present system, the energy of the flame furnace is obviously lower, and salts cannot be vaporized efficiently, leading to inevitable interference with high salt concentration. | ||
| Fig. 5 The influence of common salts on the absorbance of cadmium (0.5 µg L–1). Melting point: NaCl, 801 °C; KNO3, 333 °C; Ca(NO3)2, 500 °C (decomposition); MgSO4, 1124 °C. | ||
In the case of a NaCl matrix, another cause of loss of Cd is most probably the formation of volatile CdCl2. According to Gibb’s free energy change of chemical reaction (Cd + Cl2 → CdCl2), CdCl2 is easily formed in the temperature range of 500–1000 °C and then quickly vaporized.21 In order to validate this process, the effect of hydrochloric acid and nitric acid on the determination of Cd was investigated and the results are shown in Fig. 6. It can be seen from Fig. 6 that HCl causes a significant interference in Cd determination, while the influence of HNO3 is negligible, indicating that the formation and vaporization of CdCl2 cannot be ignored. Unfortunately, adding NH4NO3 (common chemical modifiers for Cd determination by graphite furnace AAS22) does not alleviate chloride interference, and this implies that the formation of CdCl2 occurs at the first place.
 | ||
| Fig. 6 The influence of hydrochloric acid or nitric acid on the absorbance of cadmium (1 µg L–1). | ||
3.5. Figures of merit and sample analysis
The analytical figures of merit for the present PN-FF-AAS method for Cd determination are summarized in Table 1. The sensitivity enhancement factor is found to be 730, which is defined as the slope ratio of the calibration curves for cadmium determination by PN-FF-AAS and conventional FAAS. The limit of detection (LOD, 3σ) for the determination of cadmium is found to be 15 ng L–1, and the upper linear dynamic range 2 µg L–1. As is shown in Table 2, the LOD is lower than those obtained by cloud point extraction,18fullerene23 or C18 column24 preconcentration-based TS-FF-AAS and HG-AAS,25 and comparable to those obtained by multi-wall carbon nanotubes preconcentration-based TS-FF-AAS,26 NaIO3-enhanced HG-AFS,27 and graphite furnace AAS.28 Compared with graphite furnace AAS, this procedure can easily be coupled to common preconcentration methods due to its simplicity and robustness, such as knotted reactor,29,30cloud point extraction,18,31 lab-on-valve renewable column,32,33 and flow injection/sequential injection on-line separation,34 to further improve the performance of the proposed method.| Parameter | PN-FF-AAS |
|---|---|
| Trapping time | 10 s |
| Sample volume | 300 µL |
| Calibration equation (C in µg L–1) | A = 0.1934C + 0.0015 |
| Upper linear range (µg L–1) | 2 |
| Correlation coefficient (R) | 0.9998 |
| Limits of detection (ng L–1) | 15 |
| Precision @ 0.5 µg L–1 (RSD, n = 11) | 3.2% |
| Sensitivity enhancement factor (over FAAS) | 730 |
The present method was validated by analysing several certified reference materials and the analytical results are given in Table 3. Water samples were analysed after suitable dilution, while botanical samples were firstly digested and then subjected to analysis directly or after proper dilution. A t-test shows that the analytical results by the proposed method have no significant difference with the certified values at the confidence level of 95%.
| Sample no. | Certified/µg g–1 | Found/µg g–1a |
|---|---|---|
| a Average ± one standard deviation of three trials. b ng g–1. | ||
| GBW 08607 Trace Elements in Water | 0.104 ± 0.002 | 0.098 ± 0.004 |
| GBW 08608 Trace Elements in Water | 11.2 ± 0.5b | 11.1 ± 0.4 |
| GBW (E) 080401 Trace Elements in Water | 0.100 ± 0.002 | 0.099 ± 0.004 |
| GBW 08511 Cadmium in Rice | 0.50 ± 0.02 | 0.54 ± 0.03 |
| GBW 08510 Cadmium in Rice | 2.60 ± 0.05 | 2.64 ± 0.05 |
| GBW 07605 Tea | 57 ± 10b | 54 ± 3 |
| GBW 10014 Cabbage | 35 ± 6b | 34 ± 3 |
4. Conclusion
PN-FF-AAS is simple and highly sensitive for on-line cold-trap preconcentration and determination of ultra-trace cadmium. It makes the dream of complete introduction of sample aerosol into flame AAS come true. With the low cost and easily available add-on accessories, the detectability of conventional FAAS is greatly improved to comparable to that of the more sophisticated graphite furnace AAS. Compared with BIFF-AAS or TS-FF-AAS, the sensitivity is improved by one order of magnitude for Cd determination. On the other hand, it should be pointed out that, due to lower temperature inside the flame furnace, the proposed system is more prone to salt interference. However, simple dilution can be used to minimize the salt matrix interference when necessary, due to its high sensitivity. Nevertheless, it is suitable for the determination of trace cadmium in samples of simple matrix, such as water and botanical samples. Considering the biological significance of Cd,35,36 it is promising in biological and environmental analysis for trace cadmium.Acknowledgements
Xiandeng Hou acknowledges the financial support for this project from the National Natural Science Foundation of China [No. 20675053] and Ministry of Education of China [NCET-04-0869].References
- B. Welz and M. Sperling, Atomic Absorption Spectrometry, Wiley-VCH, Weinheim, 1999 Search PubMed.
- H. Matusiewicz, Spectrochim. Acta, Part B, 1997, 52, 1711–1736 CrossRef.
- A. Gáspá and H. Berndt, Anal. Chem., 2000, 72, 240–246 CrossRef CAS.
- A. Gáspá and H. Berndt, Spectrochim. Acta, Part B, 2000, 55, 587–597 CrossRef.
- J. Davies and H. Berndt, Anal. Chim. Acta, 2003, 479, 215–223 CrossRef CAS.
- D. A. Roman-Silva, L. Rivera, T. Morales, J. Avila and P. Cortes, Int. J. Environ. Anal. Chem., 2003, 83, 327–341 CAS.
- P. C. Aleixo, D. S. Júnior, A. C. Tomazelli, I. A. Rufini, H. Berndt and F. J. Krug, Anal. Chim. Acta, 2004, 512, 329–337 CrossRef CAS.
- E. Gonzalez, R. Ahumada, V. Medina, J. Neira and U. Gonzalez, Quim. Nova, 2004, 27, 873–877 CAS.
- M. L. Brancalion and M. A. Z. Arruda, Microchim. Acta, 2005, 150, 283–290 CrossRef CAS.
- E. R. Pereira-Filho, H. Berndt and M. A. Z. Arruda, J. Anal. At. Spectrom., 2002, 17, 1308–1315 RSC.
- C. C. Nascentes, M. A. Z. Arruda, A. R. A. Nogueira and J. A. Nóbrega, Talanta, 2004, 64, 912–917 CrossRef CAS.
- C. C. Nascentes, M. Y. Kamogawa, K. G. Fernandes, M. A. Z. Arruda, A. R. A. Nogueira and J. A. Nóbrega, Spectrochim. Acta, Part B, 2005, 60, 749–753 CrossRef.
- Y. Li, Y. Jiang and X. P. Yan, Electrophoresis, 2005, 26, 661–667 CrossRef CAS.
- M. L. Brancalion, E. Sabadini and M. A. Z. Arruda, Anal. Chem., 2007, 79, 6527–6533 CrossRef CAS.
- C. Lau, A. Held and R. Stephens, Can. J. Spectrosc., 1976, 21, 100–104 CAS.
- H. Matusiewicz and M. Krawczyk, Microchem. J., 2006, 83, 17–23 CrossRef CAS.
- H. Matusiewicz, M. Kopras and R. E. Sturgeon, Analyst, 1997, 122, 331–336 RSC.
- P. Wu, Y. C. Zhang, Y. Lv and X. D. Hou, Spectrochim. Acta, Part B, 2006, 61, 1310–1314 CrossRef.
- Y. P. Ke, Q. Sun, Z. R. Yang, J. J. Xin, L. Chen and X. D. Hou, Spectrosc. Lett., 2006, 39, 29–43 CrossRef CAS.
- B. Deng, The Principles, Technologies and Applications of Atomic Absorption Spectrometry, Tsing Hua University Press, 2004 Search PubMed.
- F. Y. Wang and Z. C. Jiang, Anal. Chim. Acta, 1999, 391, 89–94 CrossRef CAS.
- X. F. Yin, B. Schlemmer and B. Welz, Anal. Chem., 1987, 59, 1462–1466 CrossRef CAS.
- M. G. Pereira, E. R. Pereira-Filho, H. Berndt and M. A. Z. Arruda, Spectrochim. Acta, Part B, 2004, 59, 515–521 CrossRef.
- E. Ivanova, H. Berndt and E. Pulvermacher, J. Anal. At. Spectrom., 2004, 19, 1507–1509 RSC.
- M. Y. Liu and S. K. Xu, At. Spectrosc., 1997, 18, 195–201 CAS.
- C. R. T. Tarley, A. F. Barbosa, M. G. Segatelli, E. C. Figueiredo and P. O. Luccas, J. Anal. At. Spectrom., 2006, 21, 1305–1313 RSC.
- G. Li, L. Wu, J. J. Xin and X. D. Hou, J. Anal. At. Spectrom., 2004, 19, 1010–1013 RSC.
- The Guide to Techniques and Applications of Atomic Spectroscopy, The Perkin Elmer Corporation, 1997, Norwalk, CT, USA, p. 5.
- Q. Y. Ye, Y. Li, Y. Jiang and X. P. Yan, J. Agric. Food Chem., 2003, 51, 2111–2114 CrossRef CAS.
- S. Cerutti, L. D. Martinez and R. G. Wuilloud, Appl. Spectrosc. Rev., 2005, 40, 71–101 CrossRef CAS.
- M. de Almeida Zezerra, M. A. Z. Arruda and S. L. C. Ferreira, Appl. Spectrosc. Rev., 2005, 40, 269–299 CrossRef.
- Y. Wang, J. H. Wang and Z. L. Fang, Anal. Chem., 2005, 77, 5396–5401 CrossRef CAS.
- Y. Wang, M. L. Chen and J. H. Wang, J. Anal. At. Spectrom., 2006, 21, 535–538 RSC.
- Y. Wang, M. L. Chen and J. H. Wang, Appl. Spectrosc. Rev., 2007, 42, 103–118 CrossRef CAS.
- A. C. Davis, P. Wu, X. F. Zhang, X. D. Hou and B. T. Jones, Appl. Spectrosc. Rev., 2006, 41, 35–75 CrossRef CAS.
- G. Henkel and B. Krebs, Chem. Rev., 2004, 104, 801–824 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2008 |
