Room-temperature self-curing ene reactions involving soybean oil†
Atanu
Biswas
*a,
Brajendra K.
Sharma
bc,
J. L.
Willett
a,
S. Z.
Erhan
b and
H. N.
Cheng
*d
aPlant Polymers Research Unit, National Center for Agricultural Utilization Research, USDA/Agricultural Research Services, 1815 N. University Street, Peoria, IL 61604, USA
bFood and Industrial Oil Research Unit, National Center for Agricultural Utilization Research, USDA/Agricultural Research Services, 1815 N. University Street, Peoria, IL 61604, USA
cDepartment of Chemical Engineering, Pennsylvania State University, University Park, PA 16802, USA
dHercules Incorporated Research Center, 500 Hercules Road, Wilmington, DE 19808-1599, USA
First published on 14th January 2008
Abstract
A mixture of soybean oil with diethyl azodicarboxylate exhibits a remarkable self-curing and thickening behavior at room temperature due to the occurrence of crosslinking ene reactions. The kinetics and the reaction mechanisms have been studied with the help of model compounds, 1H and 13C NMR, and size exclusion chromatography. The reactions are found to be most facile with linolenate, followed by linoleate, and least with oleate. The product from this reaction may be used as an environmentally friendly self-curing agent in appropriate applications.
Introduction
Self-curing, thickening, and self-gelling properties are often desirable in commercial applications.1 A number of commercial and medical products can use such properties, e.g., caulking agents, cements, coatings, adhesives, and biomaterials. For many current applications, these materials are acrylics, urethanes, poly(vinyl alcohol), cellulosic derivatives, or thermosetting polymers.As part of our work involving green chemistry, we seek to use natural bio-based materials and extend their applications. Soybean oil (SBO) is one of our favorite raw materials because it is cheap, renewable, and environmentally friendly.2 Soybean oil contains triacylglycerols with a mixture of fatty acids moieties (typically 51% linoleic acid, 25% oleic acid, 10% palmitic acid, 7% linolenic acid, and 5% stearic acid). The unsaturation in the soybean oil provides a handle to carry out further reactions. Previously we have incorporated nitrogen into the triglyceride structure through several reactions. These include the reactions of soybean oil with amines3 and diethyl azodicarboxylate (DEAD) at high temperatures,4 their hydrolysis products,5 and enzymatic reactions to produce specific derivatives.6
In this work we discovered that soybean oil and DEAD at room temperature can undergo a self-curing and thickening reaction. The modified soybean oil first forms oligomers and (with time) eventually polymerizes. This reaction permits soybean oil to be used as the raw material for a variety of novel products that require curing, thickening, or adhesive properties.
Experimental
Materials
Alkali refined soybean oil was obtained from ADM Packaged Oils, Decatur, IL and was used as received without further purification steps. All other materials were acquired from Aldrich Chemical Company.Reaction of soybean oil with DEAD
A mixture of soybean oil 5.32 g. (6.2 mmol), 3.1 g. of DEAD (18.6 mmol, 3 molar equivalents) were mixed together and allowed to sit on the lab bench. They were sampled over two weeks. The final soybean oil/DEAD adduct was obtained as a very viscous, honey-colored oil.NMR spectroscopy
All 1H and 13C NMR spectra were recorded quantitatively with a Bruker ARX-500 spectrometer (Bruker, Rheinstetten, Germany) at a frequency of 500 MHz (1H) and 125 MHz (13C) and a 5 mm dual probe. The sample solutions were prepared in deuterochloroform (CDCl3, 99.8% D, Cambridge Isotope Laboratories, Inc., Andover, MA). Standard operating conditions were used with 30° pulse angle, 3 s between pulses, and 1H decoupling.Brookfield viscosity
The dynamic viscosity at 40 °C was measured on a Brookfield (Middleboro, MA), DV-III programmable rheometer controlled by Rheocalc 2.4 software. It was equipped with a CP-40 spindle and programmed to vary the sheer rate from 0.5–10 RPM. The viscosity was determined by the software using a Bingham model. In this model, the viscosity is found from the slope of a shear rate vs. shear stress relationship. An experiment was also performed varying the shear stress instead of the shear rate, and the results were identical. The temperature of the system was controlled by a Brookfield (Middleboro, MA) TC-602 water bath.Size exclusion chromatography (SEC)
Molecular weights were measured on a PL-GPC 120 high temperature chromatograph (Polymer Laboratories, Amherst, MA) equipped with an autosampler and a built-in differential refractometer detector. Two PL gel 3 µm mixed E columns (300 mm × 7.5 mm, Polymer Laboratories) were used in series to resolve the samples. The injection volume was 100 µL. The samples were eluted using 1.00 mL min−1 flow rate of THF at 40 °C. The SEC was calibrated using a mixture of linear polystyrene standards (Mn 1700, 2450, 5050, 7000, 9200, and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 665) obtained from Polymer Laboratories (Amherst, MA), and methyl oleate (Mn 296.5), methyl linoleate (Mn 294.5), monoolein (Mn 356.5), diolein (Mn 620.9), and triolein (Mn 885.4) obtained from Aldrich Chemical Company (Milwaukee, WI).
665) obtained from Polymer Laboratories (Amherst, MA), and methyl oleate (Mn 296.5), methyl linoleate (Mn 294.5), monoolein (Mn 356.5), diolein (Mn 620.9), and triolein (Mn 885.4) obtained from Aldrich Chemical Company (Milwaukee, WI).
Discussion and results
In a systematic investigation of this room-temperature reaction system, we first undertook the studies of model systems and their reactions with diethyl azodicarboxylate (DEAD). These studies provided useful information on the reaction behavior. We then proceeded to study the room temperature reaction of DEAD with soybean oil under similar reaction conditions.1. Model reaction systems
As the first model system, we take a mixture of methyl linoleate and methyl linolenate (56 : 43 molar ratio). DEAD is added at a molar ratio of 0.88 : 1.0, relative to the fatty esters present. The reaction is monitored on the daily basis by both 1H and 13C NMR. The 1H NMR spectra for the first three days are shown in Fig. 1. | ||
| Fig. 1 1H NMR spectra of the reaction products between methyl linoleate and methyl linolenate with DEAD at room temperature. D = unreacted DEAD, D′ = reacted DEAD, L = linoleate, N = linolenate, and N′ = half-reacted N. | ||
Most of the peaks for linoleate (L) and linolenate (N) are overlapped except for the end methyls (0.885 ppm for L, and 0.970 ppm for N), and the methylenes between the double bonds (2.752 ppm for L, and 2.801 ppm for N). Soon after the addition of DEAD to the two methyl esters, only a small amount of reaction occurs as evidenced by the small reacted DEAD peak at 4.15 ppm (Fig. 1, after 15 minutes). After one day, however, most of L and N have reacted with DEAD. As in high-temperature reactions,4linoleate gives the conjugated products (L′); two conjugated products are possible, depending on the point of addition (C9 or C13). For linolenate, we see a new peak at 2.886 ppm that corresponds to the half-reacted product of N (denoted as N′ in the scheme below). This half-reacted product can react further to form other products (N″) if more DEAD is added.
It is important to note that on day 2 all DEAD has reacted whereas some linoleate and linolenate are still unreacted. Thus, there is no significant change in the spectrum in subsequent days. After day 3 the spectrum stays essentially the same, except for line-broadening, corresponding to increasing formation of dimers, tetramers, and oligomers. Quantitative estimates of the various species are obtained from 1H NMR and given in Table 1. In Scheme 1 D denotes DEAD, and D′ denotes DEAD residue.
 | ||
| Scheme 1 | ||
| Time | L | N | L′ | N′ | N″ | Unreacted DEAD |
|---|---|---|---|---|---|---|
a L and N are estimated from methyls (0.9–1.0 ppm) and methylenes (∼2.8 ppm). L′ and N′ are estimated by the decrease in the 2.8 ppm peaks. N″ is obtained by difference (N″![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N′). N–N′).
|
||||||
| Time 0 | 1.0 | 1.0 | 0 | 0 | 0 | 1.0 |
| Day 1 (within 30 min) | 0.95 | 0.92 | 0.05 | 0.06 | 0.02 | 0.88 |
| Day 2 | 0.15 | 0.04 | 0.85 | 0.04 | 0.92 | 0 |
| Day 3 | 0.15 | 0.04 | 0.85 | 0.04 | 0.92 | 0 |
The 13C NMR spectra for L + N mixture give corroborative information (Fig. 2). Basically all the DEAD has reacted fully by the second day. Because there is a deficit of DEAD relative to the amounts of olefins present, there are still some linoleate and linolenate unreacted. It is useful to note that linolenate reacts faster than linoleate.
 | ||
| Fig. 2 13C NMR spectra of the reaction products between methyl linoleate and methyl linolenate with DEAD at room temperature. | ||
As the second model system, we use the room-temperature reaction of methyl linolenate with DEAD. In this case, a surplus of DEAD is used (molar ratio of methyl linolenate versusDEAD = 1 : 4), and the reaction is carried out for 6 days. As expected, the reaction proceeds very fast. Because three double bonds are present, the reaction can occur in different ways, generating a large number of isomers. As a result, the 13C NMR spectrum is smeared in the olefin region with no distinct peaks on day 1 (Fig. 3, day 1). On day 2 or after, the only distinct peaks in the 13C spectrum (Fig. 3, day 2) are C18 (14.2 ppm), C2 (34.0 ppm), C3 (25.0 ppm), C4 (29.5 ppm), C5 (29.0 ppm), and C6 (29.0 ppm).
 | ||
| Fig. 3 13C NMR spectra of the reaction products of methyl linolenate with DEAD at room temperature. | ||
Likewise, the 1H spectra of the linolenate–DEAD reaction give a complex picture (Fig. 4). The olefin region shows an unmistakable pattern for conjugated double bonds (at 5.42, 5.65, 5.91, and 6.20 ppm); these are the same peaks found for linoleate-DEAD reactions.4 However, other peaks are also present, and the peaks are broad. After 2 days of reaction (Fig. 4, day 2), the more distinct peaks are due to protons far away from the site of reactions, e.g., methyl (0.932 ppm), H2 (2.28 ppm), H3 (1.59 ppm), H4-H6 (1.24 ppm), and methoxy (3.64 ppm).
 | ||
| Fig. 4 1H NMR spectra of the reaction products of methyl linolenate with DEAD at room temperature. | ||
2. Reactions with soybean oil
In a typical SBO–DEAD reaction, 3 mol of DEAD are reacted with 1 mol of soybean oil. Over time, the viscosity of the mixture increases. After 14 days, the mixture is so thick that we can flip the flask over and none of the material can flow downward (Fig. 5). Quantitative Brookfield viscosity data (obtained without stirring) are given in Table 2 and also shown in Fig. 6.| Hours | Days | Viscosity/mPa s |
|---|---|---|
| 0 | 0 | 97 |
| 24 | 1 | 1318 |
| 48 | 2 | 3809 |
| 120 | 5 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 741 741 |
| 144 | 6 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 927 927 |
| 168 | 7 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 565 565 |
| 192 | 8 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 590 590 |
| 216 | 9 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 063 063 |
| 288 | 12 | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 743 743 |
| 312 | 13 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 703 703 |
| 336 | 14 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 819 819 |
 | ||
| Fig. 5 Photograph of a mixture of soybean oil and DEAD at room temperature after 14 days. | ||
 | ||
| Fig. 6 Plot of Brookfield viscosity for the room temperature SBO–DEAD mixture versus time. | ||
A better understanding of the SBO–DEAD reaction can be obtained via the SEC analysis of the reaction products. The data are shown in Table 3 and plotted in Fig. 7. (It may be noted that SBO, with a formula weight of 850 gives an apparent molecular weight of 1076 with the current calibration curve.) As expected, the SBO concentration decreases steadily with time. The SBO–DEAD adduct appears, reaching a maximum on day 1, and then slowly decreasing. Starting on day 3, the dimer of the SBO–DEAD adduct is found and stays relatively unchanged in concentration. At about the same time, SBO oligomerization becomes increasingly significant to generate low-molecular-weight polymers. The high-molecular-weight SBO polymer appears more slowly. After 29 days about 26% of the reaction mixture contains high-molecular-weight SBO polymer. The Mn for the SBO high-molecular-weight polymer is about 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 Da, corresponding to a degree of polymerization of about 80.
500 Da, corresponding to a degree of polymerization of about 80.
| Sample | Reaction time/days | SBO | SBO adduct | Dimer of SBO adduct | LMW polymer | HMW polymer |
|---|---|---|---|---|---|---|
| Max RT/min | 15.48 | 14.8 | 13.58 | 13.01 | 10.17 | |
| Peak RT range/min | 15.33–16.74 | 14.00–15.34 | 13.2–14.0 | 10.47–13.23 | 9.80–10.44 | |
| Apparent M n | 1076 | 2196 | 4408 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 546 546
|
86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 520 520
|
|
| SBO | 0.0 | 100 | — | — | — | — |
| 79-2 | 1.3 | 29.2 | 63.9 | 3.3 | 3.5 | 0.0 |
| 79-4 | 3.0 | 22.3 | 55.2 | 9.5 | 12.9 | 0.0 |
| 79-5 | 4.0 | 20.0 | 51.3 | 10.0 | 18.6 | 0.0 |
| 79-6 | 7.0 | 16.8 | 40.4 | 10.4 | 29.1 | 3.4 |
| 79-x | 29.1 | 6.2 | 25.5 | 9.1 | 33.1 | 26.1 |
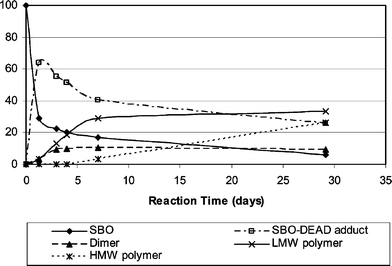 | ||
| Fig. 7 SBO–DEAD reaction progress in terms of wt% distribution with time. | ||
The kinetics of the self-curing reaction can be seen more graphically in Fig. 8. After 1.29 days of room temperature reaction, the SEC curve drifts to slightly higher molecular weights. After 4 days low-molecular-weight polymers are clearly seen (at 10–14 min). After 29 days a significant portion of the materials is high-molecular-weight polymer (at 9.0–10.5 min).
 | ||
| Fig. 8 SEC chromatograms of SBO and SBO–DEAD reaction products at different reaction times (1st from bottom = starting SBO, 2nd = after 1.29 days, 3rd = after 4 days, and 4th = after 29.13 days). | ||
The 1H NMR spectra for selected reaction times are given in Fig. 9. It should be noted that DEAD reacts somewhat slower in soybean oil than in methyl linoleate and linolenate. As before, linoleate and linolenate moieties react relatively quickly and disappear on day 2. Oleate, however, remains largely unreacted. Thus the reactivity follows the following decreasing trend: linolenate > linoleate > oleate. Quantitative estimates of all major species are given in Table 4.
| Day | L | N | L′ + N″ | Unreacted oleate | Unreacted DEAD | Reacted DEAD |
|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 0 | 0.21 | 2.7 | 0 |
| 2 | 0.05 | 0 | 0.58 | 0.21 | 0.75 | 2.0 |
| 3 | 0 | 0 | 0.54 | 0.21 | 0.40 | 2.3 |
| 4 | 0 | 0 | 0.44 | 0.21 | 0.22 | 2.5 |
| 5 | 0 | 0 | 0.44 | 0.21 | trace | 2.6 |
| 8 | 0 | 0 | 0.33 | 0.21 | 0 | 2.6 |
| 12 | 0 | 0 | 0.20 | 0.21 | 0 | 2.6 |
 | ||
| Fig. 9 1H NMR spectra of the reaction of soybean oil with DEAD at room temperature. D = unreacted DEAD, D′ = reacted DEAD, and O = oleate. | ||
Note that 58% of linoleate and linolenate have been converted to the conjugated form on day 2 (Table 4, column 4). For illustration, see Scheme 2.
 | ||
| Scheme 2 | ||
However, the amount of conjugated structures (L′ + N″) steadily decreases with time as soybean oil starts to form dimers and oligomers. This is due to the Diels–Alder reaction of olefin and diene on the fatty acid moiety of SBO–aza-dicarboxylate ester, leading to self-condensation:
Some condensation of the diene with DEAD probably also takes place. The data in Table 4 clearly shows the decrease in olefin intensities with time. Thus, the combined use of NMR and SEC provides complementary information on this reaction.
The 13C NMR spectrum of the reaction product (Fig. 10, day 1) gives the characteristic peaks for triglyceride (ester at 172.6 and 173.1 ppm, glycerol at 62.3 and 68.7 ppm), aza-carboxylate ester (156.5 ppm), diethyl (∼62, 14.3 ppm). The detailed assignments have been given elsewhere.4 It is important to note that after day 5 line-broadening starts to happen as higher molecular weight materials begin to build up. At day 16, most of the sharper features of the spectrum correspond to the unreacted oleate.
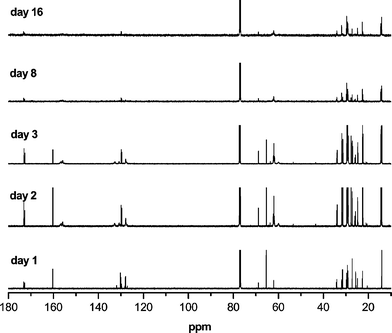 | ||
| Fig. 10 13C NMR spectra of the reaction of soybean oil with DEAD at room temperature. | ||
3. Applications
From the picture shown in Fig. 5, it is clear that this modified soybean oil can be an effective self-curing system. It is also evident that this reaction can be rendered more versatile through the formulation or suitable choice of different edible oils. For example, fish oil and linseed oil have a lot of unsaturation. Incorporation of some of these oils in the SBO–DEAD reaction will increase the degree of crosslinking and the viscosity of the final (cured) material. Since our data showed that oleate in the triglyceride oil is not substantially reacted with DEAD, olive oil can serve as a plasticizer in a formulation. Hydrogenated vegetable oils and animal fats do not react with DEAD, and they can be used as diluents in end-use formulations, e.g., to vary the rate of curing, or to control the viscosity of the final material.As soybean oil (and edible oils in general) are biodegradable and relatively cheap, this room temperature reaction may be useful in commercial applications. This is especially the case for biomaterials, where biocompatibility and toxicity of organic polymers are always of concern.
Conclusion
It is of interest that soybean oil can react with diethyl azodicarboxylate even at room temperature to form a crosslinked reaction product. The combined use of NMR, viscosity, and SEC permits a rather detailed understanding to be obtained for the reaction. The material obtained through this reaction is of interest because it is self-curing and thickening and may be an environmentally friendly alternative to organic self-curing, thickening, or adhesive systems.Acknowledgements
The authors wish to thank Janet Berfield and Kelly Utt for expert technical assistance.References
- For example
(a) C. A. G. Arrais, M. Giannini, F. A. Rueggeberg and D. H. Pashley, Oper. Dent., 2007, 32, 37–44 Search PubMed;
(b) S.-C. Wang, P.-C. Chen, J.-T. Yeh and K.-N. Chen, J. Appl. Polym. Sci., 2006, 102, 4383–4393 CrossRef CAS;
(c) J. Lee, G. R. Yandek and T. Kyu, Polymer, 2005, 46, 12511–12522 CrossRef CAS;
(d) S.-S. Hou and P.-L. Kuo, Macromol. Chem. Phys., 1999, 200, 2501–2507 CrossRef CAS;
(e)
M. Yabuta, Y. Yukawa, Y. Nakao, Self-curing resin, US Pat., 5
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 116
116![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 930, May 26, 1992.
930, May 26, 1992. - For example (a) R. D. O'Brien, Fats and Oils: Formulating and Processing for Applications, CRC Press, Boca Raton, 2nd edn, 2003 Search PubMed; (b) H. W. Lawson, Food Oils and Fats: Technology, Utilization and Nutrition, Chapman and Hall, New York, 1995 Search PubMed.
- A. Biswas, A. Adhvaryu, S. H. Gordon, S. Z. Erhan and J. L. Willett, J. Agric. Food Chem., 2005, 53, 9485 CrossRef CAS.
- A. Biswas, B. K. Sharma, J. L. Willett, K. Vermilion, S. Z. Erhan and H. N. Cheng, Green Chem., 2006, 9, 85 Search PubMed.
- A. Biswas, R. L. Shogren, J. L. Willett, S. Z. Erhan and H. N. Cheng, Polym. Prepr., 2006, 47(2), 259 CAS.
- A. Biswas, R. L. Shogren, J. L. Willett, S. Z. Erhan and H. N. Cheng, ACS Symp. Ser., 2008 Search PubMed , accepted for publication.
Footnote |
| † The use of trade, firm, or corporation names in this publication is for the information and convenience of the reader. Such use does not constitute an official endorsement or approval by the United States Department of Agriculture or the Agricultural Research Service of any product or service to the exclusion of others that may be suitable. |
| This journal is © The Royal Society of Chemistry 2008 |

