Synthesis of aluminium borate–boron oxide and binary titanium–boron and zirconium–boron oxides from metal alkoxides and (MeO)3B3O3 in non-aqueous solvents†‡
Abstract
The reaction of metal alkoxides M(OR)4 (M = Ti, Zr; R =
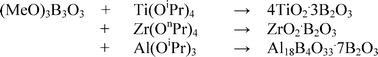
- This article is part of the themed collection: Collection of articles dedicated to Professor Ken Wade, F.R.S. in celebration of his seventy-fifth birthday

 Please wait while we load your content...
Please wait while we load your content...