Coordination chemistry of a new P,Te-centred ligand: synthesis, NMR spectra and X-ray structures of M(TePPri2NPPri2)2 (M = Zn, Cd, Hg)†‡§
Abstract
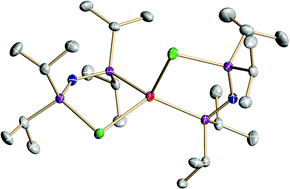
The lithiation of the monotelluride TePPri2NP(H)Pri2 (2) with BunLi at −78 °C results in the formation of the reagent [LiTePPri2NPPri2] (5), which was characterized by
- This article is part of the themed collection: Collection of articles dedicated to Professor Ken Wade, F.R.S. in celebration of his seventy-fifth birthday

 Please wait while we load your content...
Please wait while we load your content...