Abstract
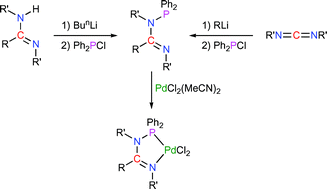
The syntheses of the cyclic N-phosphino-amidines and -guanidines Ph2PN(Pri)C(NPri2)N(Pri) (1) and Ph2PN(c-Hex)C(R)N(c-Hex) [R = piperazino (2), morpholino (3), Me (4), and Ph (5)] are reported. DFT studies have identified the preferred structures for compounds 1–5 with the E-configuration being the most stable form for the N-phosphino-amidines, while the Z-conformation is preferred for the N-phosphino-guanidines something that highlights the potential of such systems to act as κ2-P,N-chelates. The differences in donor characteristics of 2–5 have been probed through the study of their corresponding P(V)
- This article is part of the themed collection: Collection of articles dedicated to Professor Ken Wade, F.R.S. in celebration of his seventy-fifth birthday

 Please wait while we load your content...
Please wait while we load your content...