Amidinate–carboxylate complexes of dimolybdenum and ditungsten: M2(O2CR)2((NiPr)2CR′)2. Preparations, molecular and electronic structures and reactions†‡§
Abstract
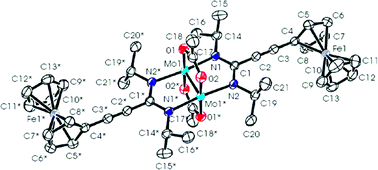
The compounds M2(O2CMe)4 and the lithium amidinates Li[(NiPr)2CR] react to give the new compounds trans-M2(O2CMe)2[(NiPr)2CR]2 where M = Mo or W and R = Me (M = Mo only), –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu, –C
CtBu, –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh and –C
CPh and –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–Fc where Fc = 1-ferrocenyl. The limitations of this type of reaction are described based on steric considerations together with the preparation and characterization of the compound Mo2(µ-O2C-9-anthracene)2[η2-(NiPr)2CMe]2. The electronic structures of the bis-amidinate–bis-carboxylate M2 complexes are described based on model compounds employing density functional theory and are correlated with the experimental observations of their physicochemical properties and in particular their observed
C–Fc where Fc = 1-ferrocenyl. The limitations of this type of reaction are described based on steric considerations together with the preparation and characterization of the compound Mo2(µ-O2C-9-anthracene)2[η2-(NiPr)2CMe]2. The electronic structures of the bis-amidinate–bis-carboxylate M2 complexes are described based on model compounds employing density functional theory and are correlated with the experimental observations of their physicochemical properties and in particular their observed ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–Ph. The kinetic lability of these new M2-containing compounds toward
C–Ph. The kinetic lability of these new M2-containing compounds toward
- This article is part of the themed collection: Collection of articles dedicated to Professor Ken Wade, F.R.S. in celebration of his seventy-fifth birthday

 Please wait while we load your content...
Please wait while we load your content...