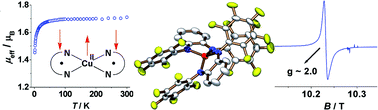
A series of homoleptic complexes with non-innocent ligands derived from N,N′-bis(pentafluorophenyl)-o-phenylenediamine (H2Fpda) are reported. [NiII(Fsbqdi)2] (1), [PdII(Fsbqdi)2] (2), [CoII(Fsbqdi)2] (3), and [CuII(Fsbqdi)2] (4) were synthesized, where (Fsbqdi)1− represents a radical anion formed by one-electron oxidation of the doubly deprotonated H2Fpda. The oxidation states of ligands and metals in complexes 1–4 were assigned by single crystal X-ray crystallography performed at low temperatures. Complex 4 is the first CuII complex where both o-phenylenediamine derived ligands are monoanionic radicals. The bulky N–C6F5 substituents force the complexes 1, 3, and 4 to adopt a twisted geometry (intermediate between square-planar and tetrahedral). The electronic structures of the neutral compounds 1–4 and of some of their cationic and/or anionic neighboring redox states were probed using EPR and UV-VIS-NIR spectroelectrochemistry. The twisted geometry of the complexes results in considerable changes in their electronic structures compared to the well known square-planar complexes while the strongly electron withdrawing N-C6F5groups have a great influence on redox properties.