A briefing on aurophilicity†
Abstract
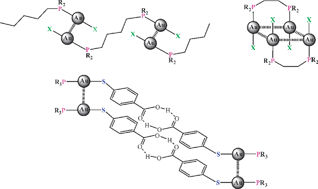
There is now compelling experimental evidence for the existence of specific intra- and intermolecular bonding between seemingly closed-shell gold(I) centers (5d10) which manifests itself in all areas of gold chemistry. This “aurophilic interaction”, which had not been predicted by conventional valence theory, was found to be associated with binding energies in some cases exceeding even those of strong hydrogen bonds and therefore to be highly significant in co-determining molecular structure and dynamics. In high-level theoretical treatments the attraction is rationalized as a “super van der Waals bonding” based on particularly strong relativistic, dispersion and correlation effects (critical review, 265 references).
- This article is part of the themed collection: Gold - Chemistry, Materials and Catalysis

 Please wait while we load your content...
Please wait while we load your content...