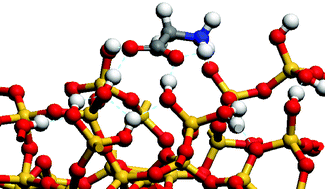
Density functional theory (DFT) periodic ab initio molecular dynamics calculations are used to study the adsorption of gaseous and microsolvated glycine on a hydroxylated, hydrophilic silica surface. The silica model is presented and the interaction of water with surface silanols is studied. The heat of interaction of water is higher with the associated silanols (be they terminal or geminal ones) studied here than with isolated silanols presented in past works. Glycine is stabilized in a parallel mode on the hydroxylated surface. Terminal silanols do not allow the stabilization of the zwitterionic form, whereas geminal silanols do. Molecular dynamics (MD) first-principle calculations show that microsolvated zwitterion glycine directly binds through the carboxylate function to a surface silanol rather than through water molecules. The adsorption mode, whether with or without additional water molecules, is parallel to the surface. The ammonium function does not interact directly with the silanol groups but rather through water molecules. Thus, the carboxylate and ammonium functions exhibit two different reactivities towards silanols. The calculated free energies, taking into account the chemical potentials of water and glycine in the gas phase, suggest the existence of a thermodynamic domain in which the glycine is present in the gas phase as well as strongly adsorbed on specific sites of the surface.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...