Isomerism and interpenetration in hydrogen-bonded network structures†
Nichola J.
Burke
a,
Andrew D.
Burrows
*a,
Mary F.
Mahon
*a and
John E.
Warren
b
aDepartment of Chemistry, University of Bath, Claverton Down, Bath, UK. E-mail: a.d.burrows@bath.ac.uk; m.f.mahon@bath.ac.uk; Fax: +44 1225 386231; Tel: +44 1225 386529
bCLRC Daresbury Laboratory, Daresbury, Warrington, UK WA4 4AD
First published on 5th September 2007
Abstract
The crystal structures of N,N-dimethylguanidinium salts based on two naphthalenedisulfonate isomers have revealed that the 1,5-disulfonate forms a three-dimensional hydrogen-bonded network whereas the 2,6-disulfonate forms an unusual structure containing interpenetrated two- and three-dimensional hydrogen-bonded networks.
With the recent upsurge of interest in network structures, interpenetration has attracted considerable attention.1 Although fascinating in its own right, interpenetration can present a problem in attempts to prepare porous materials as it inevitably leads to a reduction in pore size. There is, however, evidence that it can also impart a greater degree of stability to a metal–organic framework structure.2 Although many of the interpenetrated structures that have been reported involve metal–organic frameworks, there are also examples involving hydrogen bonded networks.3–6
The assembly of solid state structures through hydrogen bonds is an extremely topical area of chemistry.7 In one of the best illustrations of crystal engineering, Ward and co-workers have shown that guanidinium cations and sulfonate anions assemble through hydrogen bonds into hexagonal sheets,8,9 and by using disulfonates these sheets can be connected into three-dimensional arrays with predictable structures.10–12 Guanidinium disulfonates have been used to achieve the shape-selective separation of molecular isomers13 and second harmonic generation through the use of polar host frameworks.14
We have been interested in introducing substituents onto the guanidinium cation to assess the effect the concomitant loss of hydrogen bond donors has on the supramolecular structure.15–18 We found that typically N,N-dimethylguanidinium sulfonates form ribbons in the solid state in which cation–anion pairs, connected through a DD–AA interaction (graph set R22(8)) are linked into one-dimensional structures through further hydrogen bonds involving either R24(8) or R44(12) graph sets.
In order to determine whether these conclusions extend to disulfonates, we have prepared and obtained crystal structures for the naphthalenedisulfonate compounds [C(NH2)2(NMe2)]2[1,5-C10H6(SO3)2] 1‡ and [C(NH2)2(NMe2)]2[2,6-C10H6(SO3)2] 2§. The structures reveal that neither 1 nor 2 contains the anticipated hydrogen-bonded ribbons. Instead, 1 forms hydrogen-bonded sheets that are interlinked by the naphthalene groups into a three-dimensional array. In contrast, the structure of 2 contains interpenetrating two- and three-dimensional hydrogen-bonded networks.
The asymmetric unit of 1 contains a N,N-dimethylguanidinium cation and one-half of a 1,5-naphthalenedisulfonate anion, the other half of which is generated by inversion symmetry. The cations and sulfonate groups form cation–anion pairs through the anticipated DD–AA interaction involving the unsubstituted cation face. These pairs are severely twisted, with an angle of 130° between the mean cation plane and the plane of the three sulfonate oxygen atoms. The cation–anion pairs are connected into sheets (Fig. 1a) by hydrogen bonds involving the two remaining NH groups. These sheets are interlinked by the naphthalene groups into a three-dimensional array (Fig. 1b).
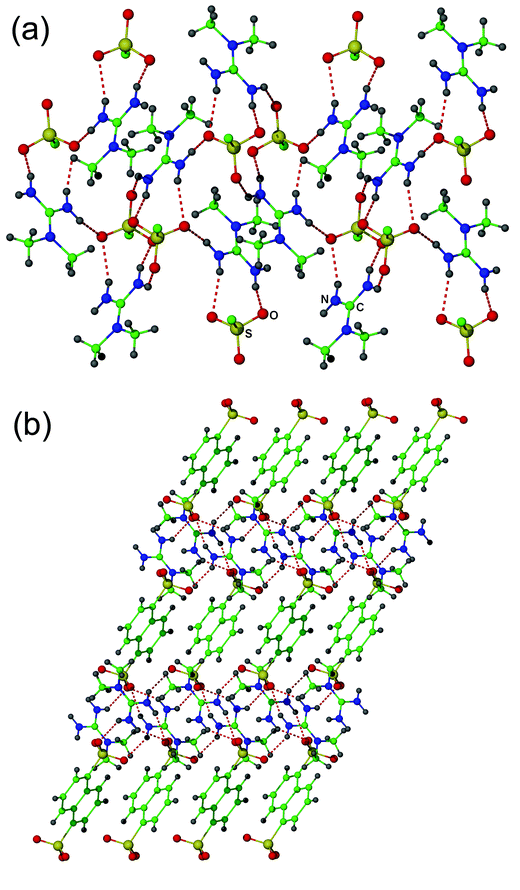 | ||
| Fig. 1 (a) Hydrogen bonded sheets in the structure of 1. (b) Interlinking of the hydrogen-bonded sheets of 1 by the naphthalene groups into a three-dimensional network. | ||
The asymmetric unit of 2 contains two independent cations and two independent anion halves, the remainder of each being generated by inversion symmetry. There are two independent and structurally distinct, interpenetrating networks present in the crystal structure of 2, one based on cations containing C(1) and anions containing S(1), and the other based on cations containing C(4) and anions containing S(2).
The cations based on C(1) and sulfonate groups based on S(1) are connected into pairs by two hydrogen bonds involving the unsubstituted cation face, but in contrast to 1 these involve only one sulfonate oxygen atom, so generate the graph set R12(6). These cation–anion pairs are connected into sheets (Fig. 2a) by hydrogen bonds involving the two remaining NH groups, giving rings described by the graph set R66(20). These sheets are linked into a three-dimensional network through the naphthalene groups, which act as bridges between sulfonates (Fig. 2b). The sulfonate groups, when connected by either the naphthalene linker or O⋯H–N–H⋯O hydrogen bonds, define a 5-connected BN (bnn) network,19 though there is distortion from trigonal bipyramidal towards square-pyramidal geometry about each node.
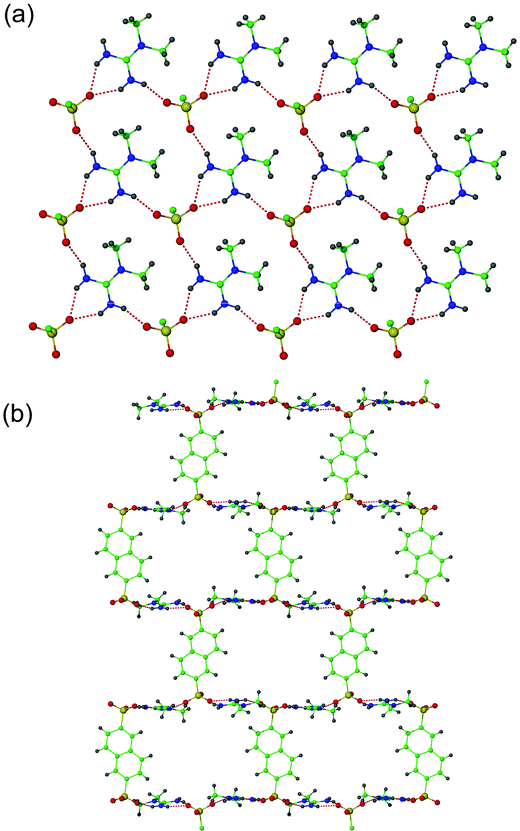 | ||
| Fig. 2 (a) Hydrogen-bonded sheets in the structure of 2. (b) Interlinking of the hydrogen-bonded sheets of 2 by the naphthalene groups into a three-dimensional network. | ||
The cation based on C(4) and sulfonate group based on S(2) form similar cation–anion pairs that contain the graph set R12(6) in contrast to R22(8), which was observed for 1. These cation–anion pairs are interlinked through further N–H⋯O hydrogen bonds to form a one-dimensional network, in which rings described by the graph set R44(16) are present. The naphthalene groups connect these chains into sheets (Fig. 3) in which the sulfonate groups define a 4,4 net.
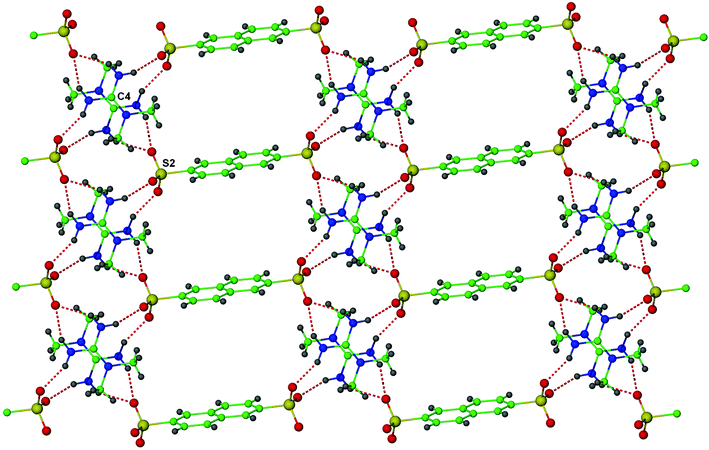 | ||
| Fig. 3 One-dimensional hydrogen bonded chains in 2 interlinked by the naphthalene groups into a two-dimensional network. | ||
The three-dimensional network containing C(1) and S(1) interpenetrates with the two-dimensional network containing C(4) and S(2) as shown in Fig. 4. This type of interpenetration is rare, with only a few reported examples involving metal–organic frameworks.20,21 Compound 2 is, we believe, the first example involving strong hydrogen bonds, though interpenetration of a two-dimensional hexagonal network and a three-dimensional α-polonium network, both constructed from C–H⋯O interactions has recently been reported.22
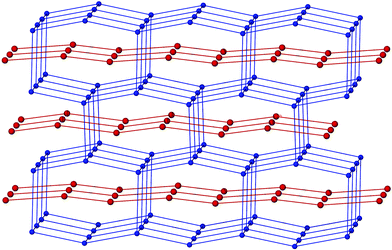 | ||
| Fig. 4 The gross structure of 2 consists of interpenetrated two- and three-dimensional networks. | ||
We thank the EPSRC for financial support.
Notes and references
- S. R. Batten, CrystEngComm, 2001, 3, 1 RSC.
- B. L. Chen, M. Eddaoudi, S. T. Hyde, M. O'Keeffe and O. M. Yaghi, Science, 2001, 291, 1021 CrossRef CAS.
- B. R. Bhogala, P. Vishweshwar and A. Nangia, Cryst. Growth Des., 2002, 2, 325 CrossRef CAS.
- S. Aitipamula, G. R. Desiraju, M. Jaskolski, A. Nangia and R. Thaimattam, CrystEngComm, 2003, 5, 447 RSC.
- A. Jayaraman, V. Balasubramaniam and S. Valiyaveettil, Cryst. Growth Des., 2005, 5, 1575 CrossRef CAS.
- M. Sarkar and K. Biradha, Cryst. Growth Des., 2006, 6, 202 CrossRef CAS.
- A. D. Burrows, Struct. Bonding, 2004, 108, 55 CAS.
- V. A. Russell, M. C. Etter and M. D. Ward, Chem. Mater., 1994, 6, 1206 CrossRef CAS.
- V. A. Russell, M. C. Etter and M. D. Ward, J. Am. Chem. Soc., 1994, 116, 1941 CrossRef.
- V. A. Russell, C. C. Evans, W. J. Li and M. D. Ward, Science, 1997, 276, 575 CrossRef CAS.
- K. T. Holman, A. M. Pivovar, J. A. Swift and M. D. Ward, Acc. Chem. Res., 2001, 34, 107 CrossRef CAS.
- R. Custelcean and M. D. Ward, Angew. Chem., Int. Ed., 2002, 41, 1724 CrossRef CAS.
- A. M. Pivovar, K. T. Holman and M. D. Ward, Chem. Mater., 2001, 13, 3018 CrossRef.
- K. T. Holman, A. M. Pivovar and M. D. Ward, Science, 2001, 294, 1907 CrossRef CAS.
- N. J. Burke, A. D. Burrows, M. F. Mahon and S. J. Teat, CrystEngComm, 2004, 6, 429 RSC.
- N. J. Burke, A. D. Burrows, M. F. Mahon and J. E. Warren, Cryst. Growth Des., 2006, 6, 546 CrossRef CAS.
- N. J. Burke, A. D. Burrows, M. F. Mahon and J. E. Warren, Inorg. Chim. Acta, 2006, 359, 3497 CrossRef CAS.
- N. J. Burke, A. D. Burrows, M. F. Mahon and J. E. Warren, CrystEngComm, 2006, 8, 931 RSC.
- M. O'Keeffe, M. Eddaoudi, H. Li, T. Reineke and O. M. Yaghi, J. Solid State Chem., 2000, 152, 3 CrossRef CAS.
- D. M. Shin, I. S. Lee, Y. K. Chung and M. S. Lah, Chem. Commun., 2003, 1036 RSC.
- J. Y. Lu and A. M. Babb, Chem. Commun., 2001, 821 RSC.
- V. S. S. Kumar, F. C. Pigge and N. P. Rath, New J. Chem., 2004, 28, 1192 RSC.
Footnotes |
| † CCDC reference numbers 654143 and 654144. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b712678f |
| ‡ Crystal data for 1: C8H13N3O3S, M = 231.27, monoclinic, space groupP21/c, a = 11.8517(7), b = 10.8528(7), c = 9.1258(5) Å, β = 111.267(2)°, U = 1093.86(11) Å3, Z = 4, ρcalc = 1.404 g cm–3, µ = 0.288 mm–1, T = 150(2) K. Reflections collected 7222, independent reflections 3028 [Rint = 0.0399]. Final R indices [I > 2σ(I)]: R1 = 0.0436, wR2 = 0.1078. |
| § Crystal data for 2: C8H13N3O3S, M = 231.27, monoclinic, space groupP21/c, a = 7.2870(6), b = 19.4830(12), c = 14.679(2) Å, β = 94.837(5)°, U = 2076.6(4) Å3, Z = 8, ρcalc = 1.479 g cm–3, µ = 0.304 mm–1, T = 170(2) K. Reflections collected 36141, independent reflections 4724 [Rint = 0.1151]. Final R indices [I > 2σ(I)]: R1 = 0.0517, wR2 = 0.1091. |
| This journal is © The Royal Society of Chemistry 2008 |
