Rigid cyanine dyenucleic acid labels†‡
Adrian
Fegan
,
Pravin S.
Shirude
and
Shankar
Balasubramanian
*
Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, UK CB2 1EW. E-mail: sb10031@cam.ac.uk; Fax: +44 1223 336913; Tel: +44 1223 336347
First published on 26th March 2008
Abstract
Cyanine dyes attached to DNA via a rigid linker show useful fluorescence and FRET properties without altering the stability of duplex DNA.
Due to their attractive fluorescence properties cyanine dyes are widely used for labelling DNA. The common method for covalently linking cyanine dyes to an oligonucleotide is via attachment to a DNA base. An incorporated nucleobase with a reactive amine is typically coupled with an activated ester of a cyanine dye to conjugate the dye via an amide (Fig. 1). There are a number of reports1–3 describing the attachment of dyes to a DNA base. All these approaches use a flexible alkane linker to attach the dye to the DNA. An important point of consideration when labelling DNA is that cyanine dyes are known to interact with DNA through non-covalent interactions.4 For example, NMR spectroscopy has shown that a Cy3 label attached via a flexible linker can stack on the end of a DNA duplex.5 Interactions between the dye and the DNA may perturb the system being studied and can also alter the fluorescent properties of the dye.
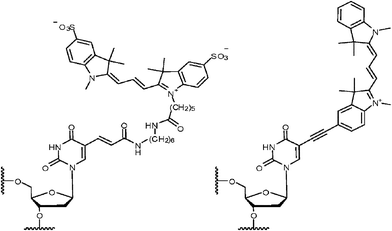 | ||
| Fig. 1 Attachment of a Cy3 dye to DNA via a common flexible linker (left) and a rigid ethynyl linker (right). | ||
Herein we report a strategy to introduce cyanine dyesvia a rigid ethynyl linker whose structure should prevent the interaction of the dye with the DNA and hence reduce the influence of the label on the system. A range of groups and functionalities, such as pyrene, have been incorporated into oligonucleotidesvia an ethynyl linker for a variety of purposes that have included electron transfer systems.6 Our approach involved the synthesis of cyanine dyes with terminal ethynyl functionalities suitable for coupling to a resin bound oligonucleotide (Scheme 1). As the C5 methyl group of thymidine is orientated into the major groove upon duplex formation, attaching a dye to this position via a rigid linker should ensure that the dye will be held away from the DNA, reducing the likelihood of interaction, providing that Watson–Crick base pairing of the associated base is not perturbed. This rigid linker restricts the mobility of the dye to rotation around the ethynyl linker, thus reducing the conformational space available to the dye. We have introduced both Cy3 and Cy5 labels with this rigid ethynyl linker and have studied their suitability to act as a Cy3–Cy5 FRET pair.
 | ||
| Scheme 1 Synthesis of the ethynyl functionalised Cy3: (i) Ac2O, pyridine, 50 °C, 5 h; (ii) trimethylsilylacetylene, PdCl2(PPh3)2, CuI, Et3N, DMF, 50 °C, 1 h; (iii) K2CO3, MeOH, DCM, 20 °C, 2 h. | ||
An iodinated Cy3 dye 1 was synthesised by reaction of a hemicyanine7 and an iodinated heterocycle (Scheme 1). The Sonogashira reaction of 1 with trimethylsilylacetylene using bis(triphenylphosphine)palladium(II) dichloride, Et3N and copper iodide furnished the protected ethynyl dye. The silyl protecting groupwas removed using K2CO3 in MeOH–DCM at room temperature and purified to give the terminal ethynyl Cy3 dye2. An analogous procedure also furnished the Cy5 ethynyl dye 3 in good yields (see ESI‡ ). With the two dyes in hand we employed a solution phase coupling to furnish the free nucleoside–dye conjugates. Both nucleoside–dye conjugates were synthesised by cross-coupling of 5-iodo-2′-deoxyuridine with the relevant dye, using bis(triphenylphosphine)palladium(II) dichloride with Et3N as base in the presence of copper(I) iodide. To introduce the ethynyl dyes into DNA a resin bound oligonucleotide containing a 5-iodo-2′-deoxyuridine at the 5′-terminus was obtained, d((IU)TA GCT CCT GAA GCG), where (IU) is 5-iodo-2′-deoxyuridine. It was produced with the 5′-O-DMTr protection on (ATDBio Ltd.). The on-resin Sonogashira cross-coupling8 was performed following the procedure described for the incorporation of 1-ethynylpyrene.6c Upon cross-coupling of the ethynyl Cy5 dye3, the crude reaction mixture was analysed by HPLC giving two peaks: one at 7.5 min (corresponding to unreacted oligo) and one at 13 min (Fig. 2). The peak at 13 min absorbed at 650 nm as well as 260 nm, showing incorporation of a Cy5 dye. Mass spectrometry confirmed the mass for the Cy5 modified oligonucleotide, Cy5-rigid (ESI‡ ). The same procedure using 2 yielded the Cy3 modified oligonucleotide, Cy3-rigid. The efficiency of couplings was found to be up to 94%, as determined by the relative integration of the peaks at 260 nm for labelled and unreacted oligonucleotides for the coupling reactions, corrected for differing extinction coefficients. While this approach has used terminal modifications, an analogous chemistry9 has been used to prepare internal and multiply labelled oligonucleotides.
 | ||
| Fig. 2 HPLC trace showing overlay of HPLC traces at 260 nm for unreacted oligonucleotide (blue line) and Cy5 coupled reaction (black line). The UV absorbance at 650 nm is shown as the red line. | ||
To explore and compare the properties of these rigid dye DNA conjugates with more conventional labelled DNA constructs that employ flexible linkers, we acquired oligonucleotidesCy3-flexible and Cy5-flexible from a commercial supplier (IBA). The flexible linker for Cy3-flexible is shown in Fig. 1 (left) and the same linker was used for Cy5-flexible. The sequences of oligonucleotidesCy3-rigid and Cy5-rigid (prepared as described earlier) were identical to Cy3-flexible and Cy5-flexible (Table 1). The unmodified analogue of the fluorescent oligonucleotide (Unlabelled) was also obtained along with the complementary strand (Comp) (Table 1).
| Name | Sequence (5′ to 3′) |
|---|---|
| Comp | CGC TTC AGG AGC TAA |
| Unlabelled | TTA GCT CCT GAA GCG |
| Cy3-rigid/flexible | T(Cy3)TA GCT CCT GAA GCG |
| Cy5-rigid/flexible | T(Cy5)TA GCT CCT GAA GCG |
To study the effect of the attached dyes on the structure and stability of DNA, circular dichroism (CD) spectroscopy and UV–thermal melting analysis were carried out. The CD spectra for Cy3-rigid:Comp and Cy5-rigid:Comp duplexes (ESI‡ ) were found to be similar to that of the unmodified duplex, suggesting the attachment of the dyes to the nucleobases does not perturb the structure of the duplex. The thermal UV–melting temperatures, Tm, of these DNA duplexescan be determined by following UV absorbance at 260 nm, which exhibited hyperchromic melting transitions. These transitions were reversible as judged by their superimposable melting and annealing curves. The Tm of both the duplexes comprising rigid labels (57 ± 1 °C) was found to be the same as that of unlabelled duplex (56 ± 1 °C) (Table 2). This suggests that the base pairing ability of the nucleobase is not affected by the attachment of the dye with a rigid linker and furthermore that the overall stability of the duplex DNA system is not perturbed by the label. For the corresponding duplexes comprising DNA with a dye attached via a flexible linker, the Tm determined was 8 to 9 °C higher than that of the unlabelled duplex (Table 2), suggesting that a cyanine label with flexible linker stabilises the duplex.
| Duplexes | T m/°C |
|---|---|
| a Conditions: 2.5 µM DNA, 10 mM sodium phosphate, pH 7.0, 100 mM NaCl and 1 mM disodium EDTA. | |
| Unlabelled:Comp | 56 ± 1 |
| Cy3-rigid:Comp | 57 ± 1 |
| Cy5-rigid:Comp | 57 ± 1 |
| Cy3-flexible:Comp | 64 ± 1 |
| Cy5-flexible:Comp | 65 ± 1 |
The fluorescence emission and excitation spectra of the different dye conjugates were measured. The free dye–nucleobase conjugate and dye–DNA conjugate of rigid Cy3 dye show a 26 nm red shift in the emission maxima, whereas an 18 nm red shift was observed for the emission spectra of both the conjugates of the rigid Cy5 dye, as compared to their corresponding free iodinated dye. The excitation maxima for both rigid Cy3 and Cy5 dyes were red shifted by 14 nm for both the conjugates. The red shift may be due to the influence of geometry or electron conjugation of ethynyl linker on the cyanine moiety. In the case of labels with the flexible linker, no such red shift was observed.
We also explored the anisotropies (r) of the dye constructs. For Cy3-rigid, r = 0.29 ± 0.01, whereas for Cy3-flexible, r = 0.22 ± 0.01. For Cy5-rigid, r = 0.28 ± 0.02 whereas for Cy5-flexible, r = 0.20 ± 0.01. The lower anisotropy values for flexible constructs may reflect that the dye label is able to move freely during its fluorescence lifetime. Both the rigid dye conjugates show no change in the anisotropy upon hybridisation of a complementary strand (Cy3-rigid:Comp, r = 0.29 ± 0.01 and Cy5-rigid:Comp, r = 0.29 ± 0.01). In contrast, flexible dye conjugates show an increase in anisotropies after hybridisation of the complementary strand (Cy3-flexible:Comp, r = 0.25 ± 0.01 and Cy5-flexible:Comp, r = 0.23 ± 0.01), signifying restricted dynamics of the fluorophore, suggesting the interaction of dye with DNA duplex stabilises the duplex as seen by their melting temperatures.
To assess the suitability of our new dye–DNA construct for FRET applications, we designed a system that employed a complementary 26 mer template strand (Template) to position the two fluorescently labelled oligonucleotides adjacent to each other (Fig. 3). The Cy3-rigid:Cy5-rigid system consisted of 1 : 1 : 1 equiv. of Template:Cy3-rigid B:Cy5-rigid (ESI‡ ). A system without the Cy5 label was also prepared. In the absence of the Cy5-rigid label (i.e. acceptor), excitation at 530 nm led to emission at 570 nm. In the presence of acceptor Cy5-rigid, reduction in the donor (Cy3-rigid B) emission and sensitized emission in acceptor (Cy5-rigid) at 670 nm were observed showing efficient FRET from the Cy3 to the Cy5 fluorophore (Fig. 4). This FRET was comparable in magnitude with the corresponding flexible dye–DNA construct (Fig. 4).
 | ||
| Fig. 3 Schematic of FRET system. | ||
 | ||
| Fig. 4 Ensemble FRET spectra of (left) Template:Cy3-flexible B:Cy5-flexible, Template:Cy3-flexible B, Template:Cy5-flexible and (right) Template:Cy3-rigid B:Cy5-rigid, Template:Cy3-rigid B, Template:Cy5-rigid with excitation at 530 nm. | ||
Our data suggest that rigid Cy3 and Cy5 labels in a DNA oligonucleotide do not perturb the stability or structure of a DNA duplex. In contrast, Cy3 and Cy5 linked via conventional flexible linkers cause the dyes to stabilise the formed duplex. The fluorescence and FRET properties of the cyanine dye labels attached to DNA via ethynyl linker suggest such constructs may be useful for fluorescence biophysics and assays in nucleic acid systems.
We thank Almac Sciences for funding (to AF) and the BBSRC for project funding. We thank Dr R. Rodriguez for careful proofreading of the manuscript.
Notes and references
- L. J. Brown, J. P. May and T. Brown, Tetrahedron Lett., 2001, 42, 2587 CrossRef CAS.
- D. M. Hammond, A. Manetto, J. Gierlich, V. A. Azov, P. M. Gramlich, G. A. Burley, M. Maul and T. Carell, Angew. Chem., Int. Ed., 2007, 46, 4184 CrossRef CAS.
- H. Yu, J. Chao, D. Patek, R. Mujumdar, S. Mujumdar and A. S. Waggoner, Nucleic Acids Res., 1994, 22, 3226 CrossRef CAS.
- B. A. Armitage, Top. Curr. Chem., 2005, 253, 55 CAS.
- D. G. Norman, R. J. Grainger, D. Uhrin and D. M. J. Lilley, Biochemistry, 2000, 39, 6317 CrossRef CAS.
- (a) D. Graham, J. A. Parkinson and T. Brown, J. Chem. Soc., Perkin Trans. 1, 1998, 1131 RSC; (b) D. J. Hurley and Y. Tor, J. Am. Chem. Soc., 2002, 124, 3749 CrossRef CAS; (c) M. Rist, N. Amann and H. A. Wagenknecht, Eur. J. Org. Chem., 2003, 13, 2498 CrossRef; (d) G. S. Jiao, T. G. Kim, M. R. Topp and K. Burgess, Org. Lett., 2004, 6, 1701 CrossRef CAS; (e) S. Jager, G. Rasched, H. Kornreich-Leshem, M. Engeser, O. Thum and M. Famulok, J. Am. Chem. Soc., 2005, 127, 15071 CrossRef.
- S. J. Mason, J. L. Hake, J. Nairne, W. J. Cummins and S. Balasubramanian, J. Org. Chem., 2005, 70, 2939 CrossRef CAS.
- S. I. Khan and M. W. Grinstaff, J. Am. Chem. Soc., 1999, 121, 4704 CrossRef CAS.
- J. Barbaric and H. A. Wagenknecht, Org. Biomol. Chem., 2006, 4, 2088 RSC.
Footnotes |
| † Dedicated to Professor Andrew B. Holmes on the occasion of his 65th birthday. |
| ‡ Electronic supplementary information (ESI) available: Synthesis, characterisation and CD. See DOI: 10.1039/b801629a |
| This journal is © The Royal Society of Chemistry 2008 |
