Novel phthalocyanine-based stopcock for zeolite L
Le-Quyenh
Dieu
a,
André
Devaux
b,
Ismael
López-Duarte
c,
M.
Victoria Martínez-Díaz
c,
Dominik
Brühwiler
*a,
Gion
Calzaferri
*b and
Tomás
Torres
*c
aInstitute of Inorganic Chemistry, University of Zürich, Winterthurer-strasse 190, 8057, Zürich, Switzerland. E-mail: bruehwi@aci.uzh.ch
bDepartment of Chemistry and Biochemistry, University of Bern, Freiestrasse 3, 3012, Bern, Switzerland. E-mail: gion.calzaferri@iac.unibe.ch
cDepartamento de Química Orgánica (C-I), Universidad Autónoma de Madrid, Cantoblanco 28049 Madrid, Spain. E-mail: tomas.torres@uam.es
First published on 11th January 2008
Abstract
We report the first phthalocyanine-based stopcock for selective adsorption to the channel entrances of zeolite L and realisation of a new electronic dipole moment coupling situation.
The supramolecular organisation of guests species inside a well-defined host material is a highly versatile principle for preparing systems exhibiting macroscopic properties.1Zeolite L has been shown to be an ideal host for the realisation of such systems.2 It is a cylindrically shaped, microporous aluminosilicate with hexagonal symmetry and a one-dimensional channel system running parallel to the cylinder axis. Due to the geometric constraints imposed by the channel structure, it is possible to obtain materials with very high concentrations of monomeric dyes. The orientation of the electronic transition dipole moments can, in some cases, be described by assuming a cone shaped distribution.3 Energy transfer materials, as illustrated in Scheme 1(A), can be prepared by sequentially incorporating different types of organic dyes.2 The channel entrances are plugged with specific types of molecules called stopcocks.4 These are composed of a rigid tail that can enter the channel and a head group that is too bulky to pass the pore opening.
 | ||
| Scheme 1 Schematic representation of an energy transfer material (A) and a possible arrangement of electronic transition dipole moments for a stopcock molecule parallel to the channel axis in two dimensions (B). The green double cone describes the orientation of the transition dipole moments of a dye incorporated into zeolite L.3 | ||
The spectral properties of dyes and stopcocks are precisely tuned to each other so that, upon selective excitation of the dyes inside, the energy travels via Förster Resonance Energy Transfer (FRET) to the stopcocks at the edges, but not back.2 These can now either re-emit this energy as luminescence from the crystal surface, or transfer it radiationlessly to a photoelectronic or a photochemical device, labelled as “Reaction centre” in Scheme 1(A). Such artificial antenna materials are of particular interest in the design of sensitised organic solar cells or fluorescent concentrators.5 Since the role of the stopcock head also includes the mediation of communication between molecules inside and outside of the zeolite crystals, it is additionally required to be fluorescent in order to extract or inject electronic excitation energy from or into the zeolite L crystals.
Considering their strong absorption and near-infrared emission, as well as their rich electrochemical properties, phthalocyanines are among the most appealing chromophores for functional energy and electron transfer materials and photovoltaic applications.6,7
The efficiency of the FRET process depends on several parameters, among them the spectral overlap integral between the emission and absorption band, as well as the distance and relative orientation of the electronic transition dipole moments of the donor–acceptor pair. FRET will be most efficient when both electronic transition dipole moments are parallel to each other. We will focus in this communication on the specifically designed novel phthalocyanine-based stopcock IL-1. Its structural formula is shown in Fig. 1(A) and consists of a phthalocyanine head bearing three tert-butyl groups as well as one rigid tetraphenylene tail. It is of great interest because it allows for the first time to realise the situation depicted in Scheme 1(B) and because the tert-butyl groups bound to the Zn-phthalocyanine head can act as an intrinsic “insulating” layer between the stopcocks and the active layer, e.g., in an organic solar cell.5
 | ||
| Fig. 1 Structural formula of stopcock molecule IL-1 (A) as well as top (B) and side (C) views of the phthalocyanine adsorbed to the channel entrances of zeolite L (van der Waals model). | ||
A top and side view of a van der Waals model of IL-1 adsorbed to the channel entrances of zeolite L is given in Fig. 1(B) and (C), respectively. The tetraphenylene tail enters the zeolite L channels if the adsorption is carried out in an appropriate solvent.8,9
The convergent strategy used to synthesise compound IL-1 is laid out in Scheme 2. The first step implies the preparation of a “reactive” phthalonitrile precursor 2 by Suzuki coupling between 4-iodophthalonitrile10 and 4′-(4,4,5,5-tetramethyl)-1,3,2-dioxaborolyl)-4-trimethylsilylbiphenyl,8 followed by the transformation of the TMS into a iodo functional group using the ICl reagent. The statistical cross-condensation of phthalonitriles 2 and 4-tert-butylphthalonitrile in the presence of ZnCl2 yielded phthalocyanine 3 in 30% yield. The subsequent palladium mediated cross coupling of 2 with the above mentioned boronic ester gave the desired compound IL-1 in 73% yield. All new compounds were spectroscopically characterized.†
 | ||
| Scheme 2 Synthesis of the Zn(II)-phthalocyanine stopcock molecule IL-1. | ||
An adsorption procedure from a n-hexane–CH2Cl2 suspension was found to be appropriate for the attachment of IL-1 to zeolite L. The fluorescence microscopy image in Fig. 2 shows the result of such an experiment at room temperature. The luminescence is concentrated at the bases of the crystals and, as expected for the arrangement shown in Scheme 1(B), it is not polarised. These results prove that the stopcocks are located at the channel entrances.
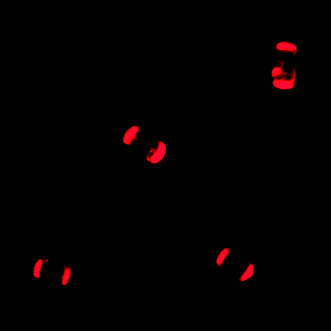 | ||
| Fig. 2 Fluorescence microscopy image of four 4.5 µm long zeolite L crystals with stopcock IL-1 adsorbed at the channel entrances. | ||
Emission (solid) and absorption (dashed) spectra of IL-1 in THF (black) and of the potential donor molecule oxonine (Ox) (light grey) are collected in Fig. 3. The dye Ox has been chosen since it has often been used successfully for synthesising energy transfer materials.2 It is well suited as donor for FRET experiments, as it can be excited in a spectral range where the stopcock IL-1 has a very weak absorption (around 550 nm). The spectral overlap integral between the emission band of Ox and the absorption band of IL-1 in THF has been determined from the spectra shown in Fig. 3(B) to be JDA = 9.76 × 10−13 cm3 M−1. Experiments with different IL-1,Ox-zeolite L samples have been made and show considerable energy transfer from Ox to IL-1.
 | ||
| Fig. 3 (A) Structural formula of oxonine (Ox) and (B) emission (solid) and absorption (dashed) spectra of a 7 × 10−7 M IL-1 solution in THF (black) and of oxonine (Ox) in zeolite L (0.025 molecules per unit cell, light grey). | ||
From this we conclude that IL-1 stopcock based energy transfer materials can be made, for which a wide range of donor or acceptor dyes is available.2 The combination of channel entrance functionalised zeolite L11 with IL-1 derivatives bearing a reactive tail end group is expected to lead to more robust materials for optoelectronic applications by providing covalent stopcock attachment.
Financial support by MEC (Spain) (grants CTQ2005-08933-BQU and CONSOLIDER-INGENIO 2010 CDS2007-00010 NANOCIENCIA MOLECULAR), by Comunidad de Madrid (Spain) (grant S–0505/PPQ/000225), by the European Commission (Marie-Curie RTN Nanomatch, Grant No. MRTN-CT-2006-035884, MRTN-CT-2006-035533, Solar n-Type and STRP 516982, Heteromolmat), by the Swiss National Science Foundation (project 200021-109185 and 200020-117591), and by the Clariant Ltd. Research Project Dye-Loaded Zeolites is acknowledged. We thank Dr Rodrigo Albuquerque for providing Fig. 1(B) and (C).
Notes and references
- (a) V. Ramamurthy, in Photochemistry in Organized and Constrained Media, ed. V. Ramamurthy, VCH publishers, New York, 1991, p. 429 Search PubMed; (b) G. Schulz-Ekloff, D. Wöhrle, B. van Duffel and R. A. Schoonheydt, Microporous Mesoporous Mater., 2002, 51, 91 CrossRef CAS; (c) K. B. Yoon, Acc. Chem. Res., 2007, 40, 29 CrossRef CAS.
- (a) G. Calzaferri, S. Huber, H. Maas and C. Minkowski, Angew. Chem., Int. Ed., 2003, 42, 3732 CrossRef CAS; (b) D. Brühwiler and G. Calzaferri, Microporous Mesoporous Mater., 2004, 72, 1 CrossRef; (c) G. Calzaferri, M. Pauchard, H. Maas, S. Huber, A. Khatyr and T. Schaafsma, J. Mater. Chem., 2002, 12, 1 RSC; (d) S. Suárez, A. Devaux, J. Bañnuelos, O. Bossart, A. Kunzmann and G. Calzaferri, Adv. Funct. Mater., 2007, 17, 2298 CrossRef CAS; (e) H. Li, Y. Wang, W. Zhang, B. Liu and G. Calzaferri, Chem. Commun., 2007, 2853 RSC; (f) A. Devaux, K. Lutkouskaya, G. Calzaferri, L.-Q. Dieu, D. Brühwiler, L. De Cola and T. Torres, Chimia, 2007, 61, 626 CrossRef CAS.
- S. Megelski, A. Lieb, M. Pauchard, A. Drechsler, S. Glaus, Ch. Debus, A. J. Meixner and G. Calzaferri, J. Phys. Chem. B, 2001, 105, 25 CrossRef CAS.
- (a) G. Calzaferri, US and EU Pat., WO 02/36490 A1, priority date 2000, granted US 2005, EU 2006 Search PubMed; (b) H. Maas and G. Calzaferri, Angew. Chem., Int. Ed., 2002, 41, 2284 CrossRef CAS.
- (a) R. Koeppe, O. Bossart, G. Calzaferri and N. S. Sariciftci, Sol. Energy Mater. Sol. Cells, 2007, 91, 986 CrossRef CAS; (b) D. Brühwiler, L.-Q. Dieu and G. Calzaferri, Chimia, 2007, 61, 820 CrossRef.
- (a) G. de la Torre, C. G. Claessens and T. Torres, Chem. Commun., 2007, 2000 RSC; (b) Phthalocyanines: Properties and Applications, ed. C. C. Leznoff and A. B. P. Lever, VCH, Weinheim, 1989, vol. 1–4 Search PubMed; (c) The Porphyrin Handbook, ed. K. M. Kadish, K. M. Smith and R. Guilard, Academic Press, New York, 2003, vol. 15–20 Search PubMed; (d) G. de la Torre, P. Vázquez, F. Agulló-López and T. Torres, Chem. Rev., 2004, 104, 3723 CrossRef CAS.
- (a) A. Morandeira, I. Lopez-Duarte, M. V. Martínez-Díaz, B. O'Regan, C. Shuttle, N. A. Haji-Zainulabidin, T. Torres, E. Palomares and J. R. Durrant, J. Am. Chem. Soc., 2007, 129, 9250 CrossRef CAS; (b) J.-J. Cid, J.-H. Yum, S.-R. Jang, Md. K. Nazeeruddin, E. Martínez-Ferrero, E. Palomares, J. Ko, M. Grätzel and T. Torres, Angew. Chem., 2007, 46, 8358 CAS; (c) D. M. Guldi, A. Gouloumis, P. Vázquez, T. Torres, V. Georgakilas and M. Prato, J. Am. Chem. Soc., 2005, 127, 5811 CrossRef CAS; (d) A. Gouloumis, D. González-Rodríguez, P. Vázquez, T. Torres, S. Liu, L. Echegoyen, J. Ramey, G. L. Hug and D. M. Guldi, J. Am. Chem. Soc., 2006, 128, 12674 CrossRef CAS; (e) A. de la Escosura, M. V. Martínez-Díaz, D. M. Guldi and T. Torres, J. Am. Chem. Soc., 2006, 128, 4112 CrossRef CAS; (f) D. González-Rodríguez, T. Torres, M. M. Olmstead, J. Rivera, M. A. Herranz, L. Echegoyen, C. Atienza Castellanos and D. M. Guldi, J. Am. Chem. Soc., 2006, 128, 10680 CrossRef CAS; (g) M. S. Rodríguez-Morgade, T. Torres, C. Atienza Castellanos and D. M. Guldi, J. Am. Chem. Soc., 2006, 128, 15145 CrossRef CAS; (h) B. Ballesteros, G. de la Torre, C. Ehli, G. M. Aminur Rahman, F. Agulló-Rueda, D. M. Guldi and T. Torres, J. Am. Chem. Soc., 2007, 129, 5061 CrossRef CAS.
- (a) O. Bossart, L. De Cola, S. Welter and G. Calzaferri, Chem.–Eur. J., 2004, 10, 5771 CrossRef CAS; (b) S. Welter, N. Salluce, A. Benetti, N. Rot, P. Belser, P. Sonar, A. C. Grimsdale, K. Müllen, M. Lutz, A. L. Spek and L. De Cola, Inorg. Chem., 2005, 44, 4706 CrossRef CAS.
- (a) R. Q. Albuquerque, Z. Popovic, L. De Cola and G. Calzaferri, ChemPhysChem, 2006, 7, 1050 CrossRef CAS; (b) R. Q. Albuquerque, A. Zabala Ruiz, L. De Cola and G. Calzaferri, Proc. SPIE, Photonics for Solar Energy Systems, 2006, 6197, 61970B-1 Search PubMed.
- S. M. Marcuccio, P. I. Svirskaya, S. Greenberg, A. B. P. Lever, C. C. Leznoff and K. B. Tomer, Can. J. Chem., 1985, 63, 3057 CAS.
- (a) S. Huber and G. Calzaferri, Angew. Chem., Int. Ed., 2004, 43, 6738 CrossRef CAS; (b) H. Li, A. Devaux, Z. Popovic, L. De Cola and G. Calzaferri, Microporous Mesoporous Mater., 2006, 95, 112 CrossRef CAS.
- (a) A. Zabala Ruiz, D. Brühwiler, T. Ban and G. Calzaferri, Monatsh. Chem., 2005, 136, 77 CrossRef CAS; (b) A. Zabala Ruiz, D. Brühwiler, L.-Q. Dieu and G. Calzaferri, in Materials Syntheses, ed. U. Schubert, N. Hüsing and R. Laine, Springer, Wien, 2008, in press Search PubMed.
- H. Maas, A. Khatyr and G. Calzaferri, Microporous Mesoporous Mater., 2003, 65, 233 CrossRef CAS.
Footnote |
† Phthalonitrile2: H NMR (300 MHz, CDCl3): δ (ppm) 8.05 (d, J = 1.6 Hz, 1H), 7.97 (dd, J = 8.3 Hz, 1.6 Hz, 1H), 7.89 (d, J = 8.3 Hz, 1H), 7.81 (d, J = 8.7 Hz, 2H), 7.69 (m, 4H), 7.37 (d, J = 8.7 Hz, 2H); C NMR (75 MHz, CDCl3): δ (ppm) 145.82, 141.61, 139.23, 138.14, 136.10, 134.08, 131.83, 131.24, 128.91, 127.95, 127.78, 116.62, 115.50, 115.39, 114.08, 94.06; HR-EI: calc. for C20H11IN2: [M]+: m/z 405.9967, found: 405.9960.Phthalocyanine3: H NMR (300 MHz, CDCl3): δ (ppm) 8.4 (br, 4H), 7.6 (br, 16H), 1.6 (br, 27H, C(CH3)3); HR-MALDI (dithranol): calc. for C56H47IN8Zn: [M]+: m/z 1022.2260, found: 1022.2275; UV/Vis (CHCl3): λmax/nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε)) = 351 (4.9), 610 (4.4), 676 nm (5.2).PhthalocyanineIL-1: H NMR (300 MHz, CDCl3): δ (ppm) 8.0 (br, 28H), 1.6 (br, 27H, C(CH3)3), 0.3 (br, 9H, Si(CH3)3); HR-MALDI (dithranol): calc. for C71H64N8SiZn: [M]+: m/z 1120.4315, found: 1120.4326; UV/Vis (CHCl3): λmax/nm (log ε)) = 351 (4.9), 610 (4.4), 676 nm (5.2).PhthalocyanineIL-1: H NMR (300 MHz, CDCl3): δ (ppm) 8.0 (br, 28H), 1.6 (br, 27H, C(CH3)3), 0.3 (br, 9H, Si(CH3)3); HR-MALDI (dithranol): calc. for C71H64N8SiZn: [M]+: m/z 1120.4315, found: 1120.4326; UV/Vis (CHCl3): λmax/nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) = 347 (4.9), 610 (4.5), 676 nm (5.2).Zeolite L crystals were synthesised and characterised as described previously.12 The potassium-exchanged form was used. Oxonine (Ox) was synthesised and purified according to a published procedure.13Ox was inserted into zeolite L by ion exchange out of H2O. A weighed amount of zeolite L crystals (typically 20 mg) was suspended in 1 ml of H2O. The desired amount of Ox was added to the suspension while vigorously stirring. The mixture was first sonicated for 15 min and then refluxed and stirred for 16 h. Afterwards the dye-loaded crystals were washed with methanol in order to remove dye molecules adsorbed on the outer surface of the crystals.For the adsorption of the phthalocyanine IL-1 to the channel ends, 10 mg of zeolite L was dispersed in 2 ml of n-hexane and sonicated for 15 min. A solution of IL-1 in CH2Cl2, containing a molar equivalent of IL-1 to the number of channel entrances, was added. The suspension was sonicated for 15 min and then stirred overnight at room temperature.Fluorescence spectra were measured with a Perkin Elmer LS 50B fluorescence spectrometer using suitable cut-off filters. Absorption spectra were recorded on a Cary 1E spectrophotometer. Fluorescence microscopy images were recorded with an Olympus BX 60 microscope equipped with a SiS CC-12 high-sensitivity CCD camera and a 100× magnification objective. ε) = 347 (4.9), 610 (4.5), 676 nm (5.2).Zeolite L crystals were synthesised and characterised as described previously.12 The potassium-exchanged form was used. Oxonine (Ox) was synthesised and purified according to a published procedure.13Ox was inserted into zeolite L by ion exchange out of H2O. A weighed amount of zeolite L crystals (typically 20 mg) was suspended in 1 ml of H2O. The desired amount of Ox was added to the suspension while vigorously stirring. The mixture was first sonicated for 15 min and then refluxed and stirred for 16 h. Afterwards the dye-loaded crystals were washed with methanol in order to remove dye molecules adsorbed on the outer surface of the crystals.For the adsorption of the phthalocyanine IL-1 to the channel ends, 10 mg of zeolite L was dispersed in 2 ml of n-hexane and sonicated for 15 min. A solution of IL-1 in CH2Cl2, containing a molar equivalent of IL-1 to the number of channel entrances, was added. The suspension was sonicated for 15 min and then stirred overnight at room temperature.Fluorescence spectra were measured with a Perkin Elmer LS 50B fluorescence spectrometer using suitable cut-off filters. Absorption spectra were recorded on a Cary 1E spectrophotometer. Fluorescence microscopy images were recorded with an Olympus BX 60 microscope equipped with a SiS CC-12 high-sensitivity CCD camera and a 100× magnification objective. |
| This journal is © The Royal Society of Chemistry 2008 |
