Strapped and other topographically nonplanar calixpyrrole analogues. Improved anion receptors
Chang-Hee Lee*a, Hidekazu Miyajia, Dae-Wi Yoona and Jonathan L. Sessler*b
aDepartment of Chemistry, Kangwon National University, Chun-Cheon 200-701, Korea. E-mail: chhlee@kangwon.ac.kr; Fax: +82 33 253 7582; Tel: +82 33 250 8490
bDepartment of Chemistry and Biochemistry and Institute for Cellular and Molecular Biology, 1 University Station, A5300, University of Texas at Austin, Austin, Texas 78712-0165, USA. E-mail: sessler@mail.utexas.edu; Fax: +1 512 471 7550; Tel: +1 512 471 5009
First published on 16th October 2007
Abstract
Calixpyrroles and related macrocycles are non-aromatic synthetic anion receptors that have attracted considerable attention in recent years. The unfunctionalized, parent calix[4]pyrrole system, also known as octamethylporphyrinogen, may be prepared in one step and in high yield from pyrrole and acetone, and is an effective anion receptor, showing a preference for fluoride, phosphate, carboxylate and chloride anions in organic media. Efforts to improve the anion binding affinity of calix[4]pyrrole and to modify its inherent selectivity have led to the synthesis of a variety of new, modified calixpyrroles. Among the most effective of these are derivatives that contain bridging “straps”. In this Feature Article, the preparation and properties of these and other topographically nonplanar calixpyrrole analogues are reviewed from the perspective of the anion recognition chemist.
 Chang-Hee Lee | Professor Chang-Hee Lee was born in 1954 in Tae-Gu, Korea. He received his PhD from the Arizona State University in 1987 and carried out postdoctoral research at the University of California at Santa Barbara. He became an Assistant Professor at Kangwon National University in 1989. He is currently a Full Professor in Chemistry. His research interests include supramolecular anion binding, unusual porphyrins, and expanded or extended porphyrin synthesis. |
 Hidekazu Miyaji | Dr Hidekazu Miyaji was born in 1968 in Kobe, Japan. He received his PhD from Shizuoka University, Japan in 1997 under the supervision of Prof. Yoshiaki Kobuke. He was a postdoctoral fellow at The University of Texas at Austin, USA with Prof. Jonathan L. Sessler from 1997 to 2001. He subsequently held several other positions and is now working as a Research Professor at Pohang University of Science and Technology (POSTECH), Korea. His current research interests are focused on anion recognition and sensing. |
 Dae-Wi Yoon | Dr Dae-Wi Yoon was born in 1974 in Incheon, Korea. He received his PhD from Kangwon National University in 2005 under the supervision of Prof. Chang-Hee Lee. He has been a postdoctoral research at The University of Texas at Austin with Prof. Jonathan L. Sessler since 2006. His current research interests include supramolecular chemistry and focused on pyrrole-based anion receptors and expanded porphyrins. |
 Jonathan L. Sessler | Professor Jonathan L. Sessler was born in Urbana, Illinois, USA on May 20, 1956. He received a BS degree (with highest honors) in chemistry in 1977 from the University of California, Berkeley. He obtained a PhD Stanford University in 1982 (supervisor: Prof. James P. Collman). He was an NSF-CNRS and NSF-NATO Postdoctoral Fellow with Prof. Jean-Marie Lehn at L'Université Louis Pasteur de Strasbourg, France. He was then a JSPS Visiting Scientist in Prof. Iwao Tabushi's group in Kyoto, Japan. He is currently the Roland K. Pettit Professor at the University of Texas at Austin. His research interests include expanded porphyrins, anion recognition, and the supramolecular chemistry of base-pairing. |
Synthetic receptors capable of effecting the selective binding and recognition of anionic substrates are of interest due to the importance of such species in areas as diverse as nuclear waste remediation, environmental chemistry and biology.1–6 For instance, an appropriate concentration of fluoride in beverages is appreciated to have a benefit in the fight against dental caries, while high concentrations of this anion can engender teeth mottling, bone density loss, or other toxic effects. Likewise, a number of diseases, including cystic fibrosis,7 Dent’s Disease and Pendred’s syndrome,8–10 are associated with poorly expressed or malfunctioning anion transport systems. Various anions, ranging from simple species, such as inorganic phosphate, to ATP, cyclic GMP, RNA and DNA, play critical roles in a range of physiological processes, including metabolism, cell signalling, and the expression of genetic information. On the environmental level, the eutrophication of waterways caused by excess nitrate or phosphate anion is a problem that can threaten both food stocks and navigation. Cyanide from gold mining operations and fluoride from semiconductor manufacture represent further specific environmental threats.11 Some radioactive nuclear waste products, particularly the pertechnetate anion (TcO4−), are also anionic and mobile in the environment, making their control and monitoring important.12 The removal of sulfate from radioactive waste streams has also been proposed as a means of improving vitrification and long-term storage.13 As a consequence, considerable effort has been devoted of late to the generation of new anion receptors, with a number of reviews and two books on the topic now being available.3,14 In this Feature article, the focus will be on structurally modified calixpyrroles, an emerging class of anion receptors that, to the best of the authors’ knowledge, has not been treated extensively in any prior review.
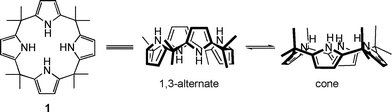
Calix[4]pyrrole or octamethylporphyrinogen (1) has been known for more than 100 years. However, it was only a bit over a decade ago that it was discovered that this easy-to-prepare heterocalixarene analogue interacts strongly with several small anions, notably fluoride, chloride and phosphate in polar aprotic media.15 In the course of these studies it was found that anion binding favours the so-called “cone” conformation, whereas the 1,3-alternate form is favoured in the absence of a strongly bound substrate (cf. structure 1). Note, however, that for the sake of simplicity in complex structures, the calix[4]pyrrole core will often be shown in a stylized flat representation.
In the years following the initial 1996 discovery of its anion binding properties, considerable effort was devoted to developing calix[4]pyrrole and its derivatives as anion receptors, extractants and sensors. Included in the progress made to date is the demonstration of so-called naked-eye anion detection,16 stationary phase-based separation of oligonucleotides,17 and anti-Hofmeister behaviour in electrochemical sensing and extraction.18 The ability of functionalized calixpyrroles to interact with neutral substrates, including nitroaromatics and C60, has also been demonstrated.19
Until 2000, when ring-expanded calixpyrrole systems started to be developed systematically, most modifications of the calix[4]pyrrole skeleton focused on functionalization of the β-pyrrolic or meso-positions. Such peripheral modifications remain among the easiest changes to effect in calixpyrrole chemistry and some of the most versatile in terms of supporting calixpyrrole-based applications. However, they are subject to important limitations, particularly with regard to potential enhancements in selectivity and affinity; even the introduction of eight highly electron-withdrawing fluorine substituents in the β-pyrrolic positions serves only to improve the anion affinities in most cases by less than a factor of 5.20 Thus, new methods are currently being sought to access calixpyrrole receptors with improved anion recognition properties. As detailed in this Feature article, one of the most effective ways of doing this is by producing strapped and other protected cavity systems, wherein the anion binding domain is both effectively defined and isolated more fully from solvent. Until recently, such systems were all but unknown in the context of calixpyrrole chemistry.
The isolation of the binding domain from the solvent matrix imparts a number of advantages in terms of substrate recognition. Firstly, such isolation can serve to enhance the affinity for a targeted guest or substrate by reducing guest–solvent and guest–counter cation interactions. Secondly, the modifications needed to effect such isolation usually produce binding domains of controlled size and shape that, in turn, generally give rise to greater inherent selectivity. Thirdly, in the specific case of calixpyrroles, these structural variations can be used to lock the conformation of the receptor into the so-called cone form, a conformation that is known to favour anion binding. Such a preorganized ‘locking’ would thus be expected to enhance further the anion affinities. These concepts, namely binding site isolation and preorganization, are not new. Indeed, they represent well established principles in the area of supramolecular chemistry whose origins can be traced back through the seminal contributions of current and early leaders in the field to the famous lock-and-key principle expressed so eloquently by Emil Fisher over a century ago.21
In spite of this generalized precedence, it was only in 2002 that the Chun-Cheon group recognized that such venerable approaches could be used to good effect in the area calixpyrrole chemistry. This led to the preparation of the so-called “strapped calixpyrroles”, a series of compounds that have now been studied extensively, often in collaboration with the Austin group. In addition, several very elegant three-dimensional oligopyrrole systems have been reported by a number of groups in addition to those of the authors. The goal of this Feature article is to provide a generalized review of ongoing efforts to develop the chemistry of topographically nonplanar calixpyrrole anion receptors and related systems. Because it reflects the current state of the art, most of the emphasis will be on receptors containing a single calix[4]pyrrole core. However, larger oligopyrrole systems are known and will be discussed as appropriate. Excluded completely from the discussion are systems based on non-pyrrolic frameworks (e.g., calixarenes), or ones containing mixed pyrrole–heterocycle skeletons (e.g., calixpyridinepyrroles). Three-dimensional systems based solely on aromatic porphyrins are also considered to lie outside the scope of this Feature article.
The intellectual progression leading to the development of protected cavity calix[4]pyrroles is summarized in Fig. 1. This schematic shows a series of sequential modifications. The simplest involves generation of a functionalized calix[4]pyrrole (2) bearing one (or more) “arms”. The second one, illustrated by generalized structure 3, embodies so-called deep cavity models, calix[4]pyrrole derivates that contain bulky meso-substituents. The third modification entails strapped systems, which are represented by structure 4, while the fourth level of complexity involves capped models, such as generic system 5. Since the degree of preorganization is increasing along the progression from 2 to 5, it might be expected that both the inherent anion selectivity and the thermodynamics of binding for appropriately sized anionic substrates would increase in a corresponding fashion. However, since (i) receptors of type 5 could prove to be too rigid, thus reducing affinity, and because (ii) their greater complexity was expected to complicate synthesis, the initial decision was to focus on strapped systems; in this case the prediction was that large enhancements in the affinity would be seen as a direct consequence of inhibiting solvent–guest interactions. In point of fact, this expectation was realized. However, prior to discussing the chemistry of strapped (and capped) calixpyrroles, it is useful to summarize what has been learned from various topographically planar models of type 2 and 3.
![Schematic representation showing various possible modifications to the basic calix[4]pyrrole core. This diagram is meant to be illustrative, not all-inclusive. Note: The calix[4]pyrrole is shown in a flattened representation in this and other complex structures for simplicity; cf. structures 1 for more accurate three-dimensional representations.](/image/article/2008/CC/b713183f/b713183f-f1.gif) | ||
| Fig. 1 Schematic representation showing various possible modifications to the basic calix[4]pyrrole core. This diagram is meant to be illustrative, not all-inclusive. Note: The calix[4]pyrrole is shown in a flattened representation in this and other complex structures for simplicity; cf. structures 1 for more accurate three-dimensional representations. | ||
A number of functionalized calixpyrroles, including many of type 2 above, have been reported over the last decade. These systems have provided the basis for many of the applications-focused advances reported to date, including colorimetric sensing, separations via attachment to a solid support, and the creation of receptors displaying enhanced selectivity. While much of this work has been reviewed elsewhere,22 a few examples are worth noting. For instance, in work from the Austin group, the pyrimidine-functionalized calix[4]pyrroles 6 and 7 were prepared and found to effect the targeted recognition and transport of complementary nucleotides.23 Likewise, when a properly functionalized calix[4]pyrrole “arm” was attached to a solid support, such as silica gel, selective separation of anionic substrates could be effected, including both mixtures of ATP, ADP and AMP and, separately, oligonucleotides on the basis of size and overall charge.
On a more fundamental level, β-pyrrole functionalization was used early on to adjust the anion binding affinities of the calix[4]pyrrole core. For instance, under conditions where the simple unfunctionalized calix[4]pyrrole (1) was reported to bind the chloride anion (studied in the form of the tetrabutylammonium (TBA) salt) with an affinity, Ka, of 1.4 × 105 M−1, as measured by ITC in dry CH3CN, the corresponding Ka value for the β-pyrrole functionalized octafluoro derivatives was 5.3 × 105 M−1.20 Such results provide clear support for the intuitively reasonable conclusion that the presence of the electron-withdrawing substituents increases the acidity of the pyrrole NH and, as a consequence, serves to enhance the anion binding affinities of the calix[4]pyrrole core. In general, however, the degree of enhancement achieved using this approach has been smaller than that attained via strapping (vide infra).
Examples of generalized system 3, namely calix[4]pyrroles with deep cavities are fewer in number.24 Typically, such systems have been prepared by “appending” substituted aryl groups onto the four meso-positions. This produces a sterically encumbered binding site that in favourable cases has been shown to display somewhat improved affinities and selectivities with regard to anion binding. However, this approach is plagued by the fact that the use of methyl ketones containing a second large substituent (e.g., an aryl group) results in the formation of up to four separate configurational isomers for the final calix[4]pyrrole. Of these, only one has all four bulky meso-aryl groups on the same side of the calix[4]pyrrole mean plane. This often leads to problems in separation and characterization.
Strapped systems, such as generalized structure 4, have an inherent advantage over deep cavity systems in that the pre-organization of the central binding domain may be better controlled. In particular, the use of a diametrically crossed strap is expected to create a well-defined binding domain, while manipulation of the strap length is expected to allow the size of the resulting ‘pocket’ to be adjusted more completely. This should translate into enhanced selectivity.
Such systems, by virtue of having two free meso-positions, may also allow for follow-up functionalization; in principle, both the normal meso and β-pyrrolic “handles”, as well as the straps themselves can be used as points for attachment to, e.g., solid supports, chromophores, or ancillary binding moieties. Thus, there is an inherent versatility to this approach that makes it quite appealing. The same holds true for fully capped systems, such as 5. These latter systems are also attractive because they might allow for more effective trapping or encapsulation of a targeted substrate. However, synthetic access to fully capped targets is far more challenging. Thus, to date most work on topographically nonplanar calixpyrroles has involved systems of general structure 4.
In designing strapped calixpyrroles of type 4, two synthetic approaches appeared attractive. These involve, respectively, either initial construction of the calixpyrrole core, followed by spanning with a strap (route A; Fig. 2) or creation of a linked precursor (incipient strap), followed by calixpyrrole ring formation (route B; Fig. 2). In point of fact, it was the second of these strategies that was first implemented successfully, with the first example of this kind of strapped calix[4]pyrrole (10) being reported by Lee and co-workers in 2002.25
 | ||
| Fig. 2 Retrosynthetic analysis of strapped system | ||
The synthesis of host 10 was accomplished in three steps (Scheme 1). The condensation of 5-hydroxy-2-pentanone with pyrrole in the presence of an acid catalyst afforded the corresponding dipyrromethane 8.
![Synthesis of the cis ester strapped calix[4]pyrrole (10).](/image/article/2008/CC/b713183f/b713183f-s1.gif) | ||
| Scheme 1 Synthesis of the cis ester strapped calix[4]pyrrole (10). | ||
The reaction of isophthaloyl dichloride with two equivalents of 8 in the presence of pyridine gave 9. Subjecting this latter acyclic precursor to acid-catalyzed condensation with acetone then afforded product 10 in 16% yield. A crystallographic analysis of 10 revealed that the calix[4]pyrrole core exists in a twisted, 1,3-alternate conformation in the solid state, as shown in Fig. 3.
A single-crystal X-ray structural analysis of the chloride complex of 10 was also carried out (cf. Fig. 4); it revealed that the anion resides within the cavity and that the calix[4]pyrrole portion of the receptor exists in a cone-like conformation. This structure also provided support for the notion the central aryl-CH is involved in anion recognition through hydrogen bonding. Specifically, the distance between the arylic-C and the bound Cl atoms was found to be 2.92 Å, a value consistent with the formation of a CH⋯Cl hydrogen bond of moderate strength.
 | ||
| Fig. 4 Single-crystal X-ray structure of the chloride anion complex of receptor 10. Hydrogen atoms and the triethylammonium counter-cation are omitted for clarity. | ||
Initial evaluations of the anion binding properties of receptor 10 were carried out in DMSO solution using standard 1H NMR spectroscopic titrations and isothermal titration calorimetry (ITC) analyses. Taken in concert, these studies revealed that the chloride and fluoride anions (studied as the corresponding TBA salts) are bound essentially irreversibly under these solution phase conditions. The affinity constant, Ka, for chloride anion could be calculated directly based on these studies and was found to be 1.0 × 105 M−1 for a 1 : 1 binding stoichiometry. Since an accurate determination of the binding constant for fluoride anion proved to be problematic by ITC, a fluoride-for-chloride competition experiment was used to estimate the affinity constant for fluoride anion; a Ka value of 3.9 × 106 M−1 was derived in this way.
Due to difficulties encountered when trying to vary the length of the ester containing strap, efforts were devoted to generating a series of strapped calix[4]pyrroles incorporating ether-based bridges of different length. This series of receptors was expected to provide insight into the effect, if any, that changes in the cavity size would have on the anion-binding selectivity of the elaborated receptor. As shown in Scheme 2, retro-Barbier type reaction of a cyclic tertiary alcohol produced the corresponding bromo-ketones in quantitative yield. m-Orcinol (14) was then reacted with the appropriate bromoketone (11, 12 and 13) to afford the key dialkoxy toluene derivatives 15, 16 and 17, respectively. Acid-catalyzed condensation of these latter ketones with pyrrole resulted in the formation of the corresponding bis-dipyrromethanes 18, 19 and 20, which afforded the desired receptors 21, 22 and 23 upon condensation with acetone.26
![Synthesis of the cis ether strapped calix[4]pyrroles, 21–23.](/image/article/2008/CC/b713183f/b713183f-s2.gif) | ||
| Scheme 2 Synthesis of the cis ether strapped calix[4]pyrroles, 21–23. | ||
The chloride and bromide anion binding affinities of the strapped systems 21–23 were determined by isothermal titration calorimetry (ITC) in dry acetonitrile using the corresponding TBA salts. Table 1 summarizes the results of these studies. This table also includes comparison data for the unsubstituted parent calix[4]pyrrole, (1), as well as the first generation ester strapped system, 10. Considered in concert, these data provide support for the notion that strapping one face of the calix[4]pyrrole core does indeed lead to systems with substantially increased chloride and bromide anion affinities. For instance, the data in Table 1 reveal that the chloride anion affinity of 21 is 25 times larger than that of 1.
| 1 | 10 | 21 | 22 | 23 | |
|---|---|---|---|---|---|
| Cl− | 1.4 × 105 | 1.4 × 106 | 3.6 × 106 | 1.4 × 106 | 1.4 × 106 |
| Br− | 3.4 × 103 | 7.5 × 103 | 3.0 × 104 | 3.1 × 104 | 1.2 × 105 |
On a more detailed level, the data presented in Table 1 serve to highlight the effects of relatively small modification in structure. For instance, within the congruent series provided by 21–23, the largest chloride anion affinity was seen with receptor 21, bearing the shortest strap (n = 1). On the other hand, the largest affinity for bromide anion was recorded in the case of the receptor containing the longest strap (23; n = 3). Since bromide is bound but poorly by calix[4]pyrrole (1), on the basis of these observations, it was concluded that not only the size of the cavity, but also its ability to stabilize aryl-CH⋯X− (X = Cl, Br) hydrogen bond interactions play key roles in regulating the observed anion affinities. While no structural data for the anion-bound forms of 21–23 is currently available, the solid state structure of the chloride anion complex of the ester strapped system 10 alluded to above provides support for the proposed aryl-CH⋯X− interactions in the solid state (cf. Fig. 4).
Evidence for these interactions was also inferred from the 1H NMR spectroscopic analyses. For instance, the central aromatic CH proton in 10, a singlet resonating at 8.65 ppm, was shifted to lower field (9.52 ppm) upon binding with chloride anion. A representative illustration of these changes is shown in Fig. 5.
 | ||
| Fig. 5 Changes in the chemical shift of key resonances for receptor 10 observed upon the addition of first tetrabutylammonium chloride (TBACl) and then excess caesium tetraphenylborate (Cs+BPh4−) in DMSO-d6 at 25 °C. | ||
Fig. 5 also serves to show that the effect of adding Cs+ to the chloride anion bound form of receptor 10 is minimal. The lack of spectroscopic changes produced as the result of this addition stands in contrast to what is seen in the case of calix[4]pyrrole (1), where evidence of Cs+X− (X = F, Cl, Br) ion pairing has recently been forthcoming.27 Apparently, in the case of the strapped system, binding of the chloride anion guest within the central cavity serves to “protect” the system from an appreciable interaction with a counter cation. Presumably, this protection extends to the solvent, with this effect accounting in part for the high relative affinities displayed by systems 10 and 21–23.
Since the basic strapping approach embodied in systems 10 and 21–23 was found to enhance the halide anion binding affinity of calix[4]pyrrole significantly, it was thought that the incorporation of additional hydrogen-bonding donor sites within the strap would serve to increase the affinity even further. In accord with such thinking, efforts were made to synthesize several strapped systems bearing isophthalate-derived diamide spacers diametrically linked to the tetrapyrrolic core.28
As shown in Scheme 3, the appropriate aminoketones were reacted with isophthaloyl dichloride to give amides 26 and 27, which were then condensed with pyrrole to afford 28 and 29. Subsequent condensation with acetone was then found to give rise to two sets of products, 30, 31 and 32, 33 corresponding to “cis” and “trans” isomeric linkages at the point of transverse attachment of the diamide containing straps.
![Synthesis of cis and trans amide strapped calix[4]pyrroles 30–33.](/image/article/2008/CC/b713183f/b713183f-s3.gif) | ||
| Scheme 3 Synthesis of cis and trans amide strapped calix[4]pyrroles 30–33. | ||
Unfortunately, analogues of 30 and 31, bearing shorter straps (i.e., n < 2), could not be obtained readily using the procedure shown in Scheme 3. However, such a target could be synthesized using an alternative method, as shown in Scheme 4. In this approach, the dipyrromethane (8) was converted into its phthalimide derivative 34. After conversion to the corresponding amine by treatment with hydrazine, immediate reaction with isophthaloyl dichloride afforded the bis-dipyrromethane 35. Acid-catalyzed condensation of 35 with acetone provided the desired product 36. In this case, no evidence for the formation of the isomeric trans-strapped system was seen. Presumably, this reflects the fact that the strap is too short to permit the formation of such an inherently strained species.
![Synthesis of the cis amide strapped calix[4]pyrrole 36.](/image/article/2008/CC/b713183f/b713183f-s4.gif) | ||
| Scheme 4 Synthesis of the cis amide strapped calix[4]pyrrole 36. | ||
The anion-binding behaviour of these receptors was investigated using proton NMR spectroscopy and isothermal titration calorimetry (ITC), with selected results being given in Table 2. As gauged from both sets of analyses, these new strapped systems were found to display affinities for both the chloride and bromide anions that are enhanced compared to those of normal, unstrapped calix[4]pyrrole but similar to those of 10 and 21–23.
| Anion | 36 | 32 | 33 |
|---|---|---|---|
| Ka/M−1 | Ka | Ka | |
| Cl− | 3.89 × 106 | 3.35 × 106 M−1 | 3.24 × 106 M−1 |
| Br− | 1.41 × 106 | 1.25 × 106 M−2 | 7.0 × 105 M−2 |
| I− | ND | 2.30 × 103 M−2 | 3.0 × 103 M−2 |
However, contrary to expectations (and in marked contrast to what was seen in the case of 21–23), no size-dependent selectivity for these anions was observed as the length of the bridging strap is varied. Such results were interpreted in terms of anion-binding processes that occur outside the central pocket defined by the strap, but that still favour strong associations as the result of the increased number of hydrogen-bonding donors provided by the amide groups.28
1H NMR spectroscopic titrations of 32 with halide anions (studied as their corresponding TBA salts) in CD3CN (1.0 mM), provided support for the above conclusion. While with fluoride anion, the saturation point in the binding curve was reached only after the addition of a full stoichiometric equivalent of the anion, in the case of chloride and bromide more complex behaviour was seen, In particular, saturation is seen after the addition of 0.5 molar equiv. of bromide anion and after the addition of ∼0.7 molar equivalents of chloride anion (Fig. 6).
 | ||
| Fig. 6 Titration of compound 32 with halide anions (studied in the form of their respective TBA salts) in CD3CN (1.0 mM). | ||
In accord with the finding that greater than 0.5 molar equiv. but less than one full stoichiometric equivalent of TBACl was required to effect a considerable change in the overall spectral features, the maximum of the Job plot (Fig. 7), corresponding to the binding of chloride anion to 32, was observed when the Cl− mole fraction was 0.4.
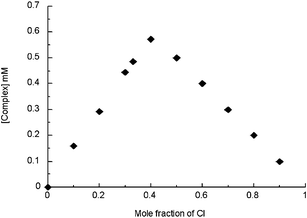 | ||
| Fig. 7 Job plot corresponding to the titration of compound 32 with chloride anion (as TBACl) in CD3CN. | ||
Such a mole fraction value, which was found to be entirely reproducible, is not consistent with the formation of a 2 : 1 (receptor : anion) binding stoichiometry Nor, is it consistent with the formation of a 1 : 1 complex. However, it may indicate the existence of equilibrium between a 1 : 1 and 2 : 1 (receptor : anion) complex as shown in Scheme 5. In particular, it is suggested that at high relative receptor concentrations (i.e., after the addition of only a small amount of TBACl), a 2 : 1 (receptor : anion) complex forms with 32 that then dissociates to produce a 1 : 1 complex as the relative concentration of the anionic substrate increases. Thus, the measured stability constant for chloride anion must actually reflect the sum of two binding equilibria (represented by constants, K1 and K2), corresponding to the formation of the 1 : 1 and 2 : 1 complexes, which cannot be calculated separately under the experimental conditions. In the case of fluoride, a complex of 1 : 1 stoichiometry is formed, whereas in the case of bromide it is the 2 : 1 species (receptor : anion ratio) that predominates.
 | ||
| Scheme 5 Proposed binding modes for the halide anion complexes of 32. | ||
In spite of the complexities of the chloride anion binding stoichiometry, a possible application of these isophthalate-derived diamide strapped systems for the detection of anions was demonstrated. In the mixed solvent system 1% H2O–CD3CN the chemical shifts of the pyrrolic NH protons observed in the 1H NMR spectrum of 32 were found to be correlated uniquely with the nature of the anions in question (e.g. F = 11.9, Cl−= 10.3, Br− = 10.0 ppm). Moreover, by using the relative integral of the chloride-bound NH peak, which was in slow exchange with the NH signal seen in the absence of an added anion, it proved possible to determine semi-quantitatively the concentration of chloride anion in both sea water and drinking water (see Fig. 8).29
![300 MHz 1H NMR spectra of (a) diamide-strapped calix[4]pyrrole (32) (1 mM) + TBABr (1 mM) + TBACl (1 mM); (b) 32 (1 mM) + TBABr (1 mM) + TBACl (1 mM) + TBAF (1 mM) in CD3CN; (c) 32 (1 mM) in 495 μL of CD3CN + 5 μL of sea water (collected at Incheon, Korea).](/image/article/2008/CC/b713183f/b713183f-f8.gif) | ||
| Fig. 8 300 MHz 1H NMR spectra of (a) diamide-strapped calix[4]pyrrole (32) (1 mM) + TBABr (1 mM) + TBACl (1 mM); (b) 32 (1 mM) + TBABr (1 mM) + TBACl (1 mM) + TBAF (1 mM) in CD3CN; (c) 32 (1 mM) in 495 μL of CD3CN + 5 μL of sea water (collected at Incheon, Korea). | ||
A new strapped calix[4]pyrrole, 38, containing a fluorophore as a part of the strap, was also synthesized in 2005.30 It was prepared from 5,7-dihydroxy-4-methylcoumarin via the dipyrromethane analogue 37 (Scheme 6) and follow-up condensation with acetone gave the coumarin-containing receptor 38.
![Synthesis of coumarin strapped calix[4]pyrrole 38.](/image/article/2008/CC/b713183f/b713183f-s6.gif) | ||
| Scheme 6 Synthesis of coumarin strapped calix[4]pyrrole 38. | ||
Association constants with various anions were determined using both fluorescence titration and isothermal titration calorimetry (ITC). Gratifyingly, the association constants obtained by these two different methods were found to be in good agreement with one another (Table 3).
An interesting feature of receptor 38 is that its fluorescence emission properties could be controlled via the addition of appropriate cations and anions (Fig. 9). In particular, it was found that the fluorescence intensity could be enhanced via the addition of sodium cations. Presumably, this reflects the fact that these latter species bind to the carbonyl moiety in coumarin, thereby turning off an inherent photoinduced electron transfer (PET) quenching process. In contrast, the fluorescence intensity of 38 could be reduced via the addition of anions that are known to bind within the calix[4]pyrrole core.30 Such systematic, substrate-dependent changes in fluorescence emission intensity means that this receptor acts as a rudimentary supramolecular logic device.
 | ||
| Fig. 9 Schematic representation of the proposed interactions between 38 and sodium cation and chloride anion. (a) The Na+ cation is thought to bind to the coumarin carbonyl oxygen atom, thereby inhibiting an inherent PET quenching process. (b) In contrast, the binding of chloride anion (Cl−) activates a different PET mode and serves to quench the fluorescence. | ||
Another fluorogenic model system, 41, a strapped calix[4]pyrrole bearing an acridine moiety, has been synthesized recently.31 The acridine moiety is of particular interest because it can act not only as a fluorophore, but also as a proton acceptor. The synthesis of 41 is shown in Scheme 7. Here, acid catalyzed condensation of 39 with pyrrole gave the bis-dipyrromethane derivative 40, which was then condensed with acetone to afford the desired receptor 41.
![Synthesis of the acridine strapped calix[4]pyrrole 41.](/image/article/2008/CC/b713183f/b713183f-s7.gif) | ||
| Scheme 7 Synthesis of the acridine strapped calix[4]pyrrole 41. | ||
Quantitative analyses of the solution phase chloride and bromide anion binding properties of 41 were made using ITC. The resulting association constants, determined in reagent grade CH3CN at 30 °C, corresponding to the formation of the chloride and bromide complexes of 41, were 2.41 × 107 M−1 and 6.81 × 104 M−1 for Cl− and Br−, respectively. Receptor 41 thus shows a relatively high selectivity factor of ∼350 for chloride anion over bromide anion.
In addition to generating sensing systems based on various built-in or appended chromophores, effort has been devoted to the preparation of chiral, nonracemic calix[4]pyrroles via the use of appropriately chosen straps. Such systems would be potentially useful in the recognition and, potentially, separation of various anion-containing enantiomeric species, including amino acids eventually.
With these considerations in mind, a pair of chiral calix[4]pyrroles, 45R and 45S, bearing an (R) or (S)-Binol (1,1′-bi(2-naphthol)) derived diether strap on one side of the tetrapyrrolic core, was prepared.32 The synthesis is summarized in Scheme 8. 6-Bromo-2-hexanone was reacted with (R)-(+)-1,1′-bi(2-naphthol) (42R) or (S)-(−)-1,1′-bi(2-naphthol) (42S) to afford the bisketones 43R and 43S, respectively. Compounds 43R and 43S were then condensed with pyrrole in the presence of a catalytic amount of trifluoroacetic acid to give dipyrromethanes 44R and 44S. Condensation of the resulting dipyrromethanes with neat acetone in the presence of a catalytic amount of BF3·OEt2 afforded the desired chiral receptors 45R and 45S.
![Synthesis of chiral, nonracemic Binol strapped calix[4]pyrroles 45R and 45S.](/image/article/2008/CC/b713183f/b713183f-s8.gif) | ||
| Scheme 8 Synthesis of chiral, nonracemic Binol strapped calix[4]pyrroles 45R and 45S. | ||
The CD spectra of the two enantiomeric Binol-strapped calix[4]pyrroles 45R and 45S were found to be nearly mirror images of one another (Fig. 10). The resulting system 45S were found to bind the chiral carboxylate anions (R)-2-phenylbutyrate or (S)-2-phenylbutyrate (studied as the tetrabutylammonium salts) with high affinity in acetonitrile. In accord with design expectations, the association constants (Ka) were ca. 10 times larger for the (S)-guest–(S)-host pair than in the case of the corresponding (R)-guest–(S)-host combination.
 | ||
| Fig. 10 CD spectra of 45R (R) and 45S (S) in acetonitrile (1 × 10−5 M at 25 °C). | ||
Proposed binding modes for the complex formed between the Binol-strapped calix[4]pyrrole (45S) and (R)-2-phenylbutyrate and (S)-2-phenylbutyrate, respectively, are shown in Fig. 11. These depictions serve to illustrate the intuitively appealing assumption that the lower association constant observed for the combination of 45S and (R)-2-phenylbutyrate reflects unfavourable steric interactions between the chiral, nonracemic receptor and the phenyl group of the guest. By contrast, the larger association constant observed for 45S and (S)-2-phenylbutyrate can be rationalized in terms of favourable edge-centered interactions between one of the receptor naphthyl groups and the phenyl group of the bound substrate.
 | ||
| Fig. 11 Proposed binding modes for the diastereomeric complexes formed between receptor 45S and (S)-2-phenylbutyrate anion (left) and (R)-2-phenylbutyrate anion (right) (studied as the tetrabutylammonium salt) showing the relevant intermolecular interactions. Note the destabilizing steric interactions that are present in the latter structure. | ||
With chiral Binol-derived strapped calix[4]pyrroles bearing additional hydrogen bonding donor sites on the strap, even higher selectivity would be expected. To test this hypothesis, the new (R) and (S)-BINAP (1,1′-bi(2-naphthyl-2,2′-diamine) derived diamide-strapped calix[4]pyrrole (46) were synthesized and studied.
As expected, this new chiral host displayed enhanced selectivity in the binding of the enantiomeric carboxylate anions, (S)-phenylbutyrate and (R)-phenylbutyrate (studied as the tetrabutylammonium salts). In fact, the association constants (Ka) were found to be ca. 20 times larger in the case of the (S)-guest than in the case of the (R)-guest.29 Construction of a pre-organized receptor containing both hydrogen bonding donor sites and a Lewis acidic metal ion centre is also something that can be conceived within the strapped calixpyrrole paradigm. Since the putative metal-centre could act as an electron-pair acceptor or as a redox active site, the ensuing combination of anion recognition and metal cation coordination could lead to enhancements in substrate binding or selectivity. These potential benefits provided the incentive to prepare the calix[4]pyrrole-metalloporphyrin conjugates 47–49.33
Anion binding studies revealed that receptors 47–49 bound fluoride anion strongly in organic solvents, with none of the compounds displaying any appreciable affinity for Cl−, Br− or I−. Presumably, this strong size selectivity is imposed by the combination of the Lewis acid and hydrogen bonding recognition functionality present within this preorganized receptor.34 Consistent with this conclusion were the results of standard 1H NMR spectroscopic titrations and associated Job plots, which provided support for the bound fluoride anion residing within the cavity.
While still at an early stage of development, some effort has been made to extend the principle of strapping and binding site isolation beyond the realm of simple calix[4]pyrroles; to date, this has been done via the synthesis of the capped calix[6]pyrroles 50 and 51.35 Interestingly, these larger systems, like their smaller congeners discussed above, were found to display appreciable selectivity for the fluoride anion, at least in organic media.
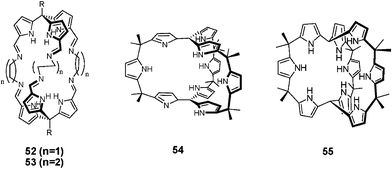
A different approach to achieving non-planar topographies involves constructing cryptand-like systems. The first such system to be characterized structurally was the imine-linked tripyrromethane dimer 52·53 reported by P. Beer in 2001.36 In this case, the neutral substrate, ethylenediamine, was found within the cavity, with the corresponding solution phase binding affinity being 1500 M−1 in CDCl3. Slightly thereafter, the Sessler group reported the synthesis and structure of a ‘3-D calixpyrrole’54 and its oxidized analogue 55.37 Neither of these latter systems contains a cavity-like void space. As a result, only solvent molecules, such as water and dichloromethane, were found trapped inside. However, in the case of 54, outside binding of anions was observed.
A system that in some respects is closer to being a real cryptand-like calix[4]pyrrole was recently synthesized by the Lee group.38 The product in question 56 was designed so as to incorporate additional pyrrolic NH donor functionality within a strapped calix[4]pyrrole framework.
Quantitative analyses of the solution phase fluoride anion binding properties of 56 were performed using isothermal titration calorimetry (ITC) in DMSO. It was found that for Ka values measured were substantially larger than for other analogous strapped systems. The titration isotherms also revealed the presence of a second event after the initial heat evolving process (Fig. 12). This second event is ascribed to the binding of a second fluoride anion by the two pyrrole NH protons of the bridging strap. Evidence of such ancillary binding interactions was not found in the case of chloride or acetate anion. The calculated Ka1 value for fluoride anion binding was 1.28 × 108 M−1, while the calculated Ka2, corresponding to the proposed second fluoride anion binding event, was 1.35 × 105 M−1, both in DMSO at 25 °C. The 1 : 1 affinity constants for chloride and acetate anion binding in the same solvent were 3.27 × 107 M−1 and 9.35 × 106 M−1, respectively. These values are noteworthy for being substantially larger than those seen for any other neutral pyrrolic anion receptor, especially in light of the fact that the solvent (spectral grade DMSO) was not subject to any special drying. It thus appears as if the use of pyrrole-bearing straps provides a particularly effective means of enhancing the anion binding affinities of strapped calix[4]pyrrole anion receptors.
 | ||
| Fig. 12 ITC titration curve for the interaction of receptor 56 with tetrabutylammonium fluoride in DMSO at 25 °C. The concentration of 56 is 0.754 mM. | ||
Conclusions
While completely unknown until recently, the chemistry of strapped calix[4]pyrroles has blossomed in the past several years. Although less well developed, the important strides have been made to generate other classes of topographically non-planar oligopyrroles. Taken in concert, these systems demonstrate enhanced anion affinities and improved selectivities relative to analogous compounds with a lower level of preorganization. For instance, the affinity constants measured in the same solvent system (e.g., acetonitrile) are seen to increase upon the introduction of straps, increasing the number of hydrogen bonding donors, or in the most general terms, upon going from 2D to 3D as far as the receptor topography is concerned. Such a global conclusion leads us to suggest that the chemistry of pre-organized calixpyrroles, which is still in its early days, is rife with possibilities and that ever more elaborate systems, containing different kinds of straps, caps, or oligopyrrolic cores, will allow for even greater diversity and selectivity in terms of substrate recognition.Acknowledgements
This work was supported by the Korea Science and Engineering Foundation (KOSEF) (grant no. R01-2006-000-10001-0) and the U. S. National Institutes of Health (grant no. GM58907).References
- A. Gopalan, O. Zincircioglu and P. Smith, Radioact. Waste Manage. Environ. Restor., 1993, 17, 161 Search PubMed.
- H. Brim, S. C. McFarlan, J. K. Fredrickson, K. W. Minton, M. Zhai, L. P. Wackett and M. J. Daly, Nat. Biotechnol., 2000, 18, 85 CrossRef CAS.
- J. L. Sessler, P. A. Gale and W. S. Cho, Synthetic Anion Receptor Chemistry, Royal Society of Chemistry, Cambridge, 2006 Search PubMed.
- P. Chakrabarti, J. Mol. Biol., 1993, 234, 463 CrossRef CAS.
- M. A. Van Kuijck, R. A. M. H. Van Aubel, A. E. Busch, F. Lang, G. M. Russel, R. J. M. Bindels, C. H. Van Os and P. M. T. Deen, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 5401 CrossRef CAS.
- B. J. Calnan, B. Tidor, S. Biancalana, D. Hudson and A. D. Frankel, Science, 1991, 252, 1167 CAS.
- M. P. Anderson, R. J. Gregory, S. Thompson, D. W. Souza, S. Paul, R. C. Mulligan, A. E. Smith and M. J. Welsh, Science, 1991, 253, 202 CAS.
- O. Devuyst, P. T. Christie, P. J. Courtoy, R. Beauwens and R. V. Thakker, Hum. Mol. Genet., 1999, 8, 247 CrossRef CAS.
- J. I. Wemeau, V. Vlaeminck-Guillem, F. Dubrulle, V. Dumur and C. Vincent, Presse Med., 2001, 30, 1689 Search PubMed.
- J. Rutishauser and P. Kopp, Eur. J. Endocrinol., 1998, 138, 623 Search PubMed.
- A. Muezzinoglu, Crit. Rev. Environ. Sci. Technol., 2003, 33, 45 Search PubMed.
- R. Colton, The Chemistry of Rhenium and Technetium, John Wiley and Sons, Ltd, Interscience Publishers, New York, 1st edn, 1965 Search PubMed.
- (a) G. J. Lumetta, in Fundamentals and Applications of Anion Separations, ed. B. A. Moyer and R. P. Singh, Kluwer Academic/Plenum, New York, 2004, pp. 107–114 Search PubMed; (b) B. A. Moyer, L. H. Delmau, C. J. Fowler, A. Ruas, D. A. Bostick, J. L. Sessler, E. Katayev, G. D. Pantos, J. M. Llinares, M. A. Hossain, S. O. Kang and K. Bowman-James, Adv. Inorg. Chem., 2006, 59, 175–204.
- The Supramolecular Chemistry of Anions, ed. A. Blanchi, K. Bowman-James E. Garcia-Espana, Wiley-VCH, New York, 1997 Search PubMed.
- P. A. Gale, J. L. Sessler, V. Král and V. Lynch, J. Am. Chem. Soc., 1996, 118, 5140 CrossRef CAS.
- H. Miyaji, W. Sato and J. L. Sessler, Angew. Chem., Int. Ed., 2000, 39, 1777 CrossRef CAS.
- J. L. Sessler, P. A. Gale and J. W. Genge, Chem.–Eur. J., 1998, 4, 1095 CrossRef CAS.
- (a) V. Král, J. L. Sessler, T. V. Shishkanova, P. A. Gale and R. Volf, J. Am. Chem. Soc., 1999, 121, 8771 CrossRef CAS; (b) T. G. Levitskaia, M. Marquez, J. L. Sessler, J. A. Shriver, T. Vercouter and B. A. Moyer, Chem. Commun., 2003, 2248 RSC.
- K. A. Nielsen, W. S. Cho, G. H. Sarova, B. M. Petersen, A. D. Bond, J. Becher, F. Jensen, D. M. Guldi, J. L. Sessler and J. O. Jeppesen, Angew. Chem., Int. Ed., 2006, 45, 6848 CrossRef CAS.
- (a) J. L. Sessler, P. Anzenbacher, Jr., J. A. Shriver, K. Jursíková, V. M. Lynch and M. Marquez, J. Am. Chem. Soc., 2000, 122, 12061 CrossRef CAS; (b) J. L. Sessler, W. S. Cho, D. E. Gross, J. A. Shriver, V. M. Lynch and M. Marquez, J. Org. Chem., 2005, 70, 5982 CrossRef CAS.
- (a) E. Fischer, Ber. Dtsch. Chem. Ges., 1894, 27, 2985 CrossRef CAS; (b) J-M. Lehn, Supramolecular Chemistry—Concepts and Perspectives Chemistry, VCH, Weinheim, Germany, 1995 Search PubMed; (c) J. W. Steed and J. L. Atwood, Supramolecular Chemistry, Wiley, New York, 2000 Search PubMed.
- J. L. Sessler, S. Camiolo and P. A. Gale, Coord. Chem. Rev., 2003, 240, 17 CAS.
- (a) J. L. Sessler, D. E. Gross, W. S. Cho, V. M. Lynch, F. P. Schmidtchen, G. W. Bates, M. E. Light and P. A. Gale, J. Am. Chem. Soc., 2006, 128, 12281 CrossRef CAS; (b) J. L. Sessler, V. Král, T. V. Shishkanova and P. A. Gale, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 4848 CrossRef CAS.
- (a) J. L. Sessler, P. Anzenbacher, Jr., H. Miyaji, K. Jursíková, E. R. Bleasdale and P. A. Gale, Ind. Eng. Chem. Res., 2000, 39, 3471 CrossRef CAS; (b) P. Anzenbacher, Jr., K. Jursíková, V. M. Lynch, P. A. Gale and J. L. Sessler, J. Am. Chem. Soc., 1999, 121, 11020 CrossRef; (c) S. Camiolo and P. A. Gale, Chem. Commun., 2000, 1129 RSC.
- D. W. Yoon, H. Hwang and C. H. Lee, Angew. Chem., Int. Ed., 2002, 41, 1757 CrossRef CAS.
- C. H. Lee, H. K. Na, D. W. Yoon, D. H. Won, W. S. Cho, V. M. Lynch, S. V. Shevchuk and J. L. Sessler, J. Am. Chem. Soc., 2003, 125, 7301 CrossRef CAS.
- J. L. Sessler, D. E. Gross, W. S. Cho, V. M. Lynch, F. P. Schmidtchen, G. W. Bates, M. E. Light and P. A. Gale, J. Am. Chem. Soc., 2006, 128, 12281 CrossRef CAS.
- C. H. Lee, J. S. Lee, H. K. Na, D. W. Yoon, H. Miyaji, W. S. Cho and J. L. Sessler, J. Org. Chem., 2005, 70, 2067 CrossRef CAS.
- H. Miyaji and C. H. Lee, unpublished results.
- H. Miyaji, H.-K. Kim, E.-K. Sim, C.-K. Lee, W. S. Cho, J. L. Sessler and C.-H. Lee, J. Am. Chem. Soc., 2005, 127, 12510 CrossRef CAS.
- S.-D. Jeong, J. Yoo, H. K. Na, D. Y. Chi and C.-H. Lee, Supramol. Chem., 2007, 19, 271 CrossRef CAS.
- H. Miyaji, S. J. Hong, S. D. Jeong, D. W. Yoon, H. K. Na, S. J. Hong, S. Ham, J. L. Sessler and C. H. Lee, Angew. Chem., Int. Ed., 2007, 47, 2508 CrossRef.
- P. K. Panda and C. H. Lee, Org. Lett., 2004, 6, 671 CrossRef CAS.
- P. K. Panda and C. H. Lee, J. Org. Chem., 2005, 70, 3148 CrossRef CAS.
- D. W. Yoon, S. D. Jeong, M. Y. Song and C. H. Lee, Supramol. Chem., 2007, 19, 265 CrossRef CAS.
- O. D. Fox, T. D. Rolls, P. D. Beer and M. G. B. Drew, Chem. Commun., 2001, 1632 RSC.
- (a) C. Bucher, R. S. Zimmerman, V. Lynch and J. L. Sessler, J. Am. Chem. Soc., 2001, 123, 9716 CrossRef CAS; (b) C. Bucher, R. S. Zimmerman, V. Lynch and J. L. Sessler, Chem. Commun., 2003, 1646 RSC.
- D. W. Yoon and C. H. Lee, unpublished work.
| This journal is © The Royal Society of Chemistry 2008 |