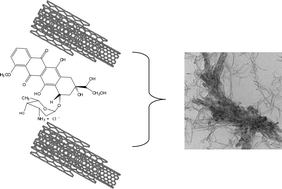
Multiwalled carbon nanotube–doxorubicin supramolecular complexes for cancer therapeutics
Abstract
Multiwalled
- This article is part of the themed collections: ChemComm contributions to the United Nations Sustainable Development Goals and ChemComm 60th Anniversary Historic Papers from the United Kingdom

 Please wait while we load your content...
Please wait while we load your content...