Investigation of the interactions between silver nanoparticles and Hela cells by scanning electrochemical microscopy†‡
Zhong
Chen
a,
Shubao
Xie
a,
Li
Shen
a,
Yu
Du
a,
Shali
He
a,
Qing
Li
a,
Zhongwei
Liang
a,
Xin
Meng
a,
Bo
Li
a,
Xiaodong
Xu
a,
Hongwei
Ma
b,
Yanyi
Huang
b and
Yuanhua
Shao
*a
aBeijing National Laboratory for Molecular Sciences, Institute of Analytical Chemistry, College of Chemistry and Molecular Engineering, Peking University, Beijing, 100871, China
bCollege of Engineering, Peking University, Beijing, 100871, China. E-mail: yhshao@pku.edu.cn; Fax: +86-10-62751708; Tel: +86-10-62759394
First published on 29th July 2008
Abstract
The interactions between Hela cells and silver nanoparticles (AgNPs) have been studied by scanning electrochemical microscopy (SECM) with both IrCl62−/3− and Fe(CN)63−/4− as the dual mediators. IrCl62−, which can be produced in situ and react with AgNPs, is used as the mediator between the AgNPs on the cells and the SECM tip. Another redox couple, Fe(CN)63−/4−, which has a similar hydrophilicity to IrCl62−/3−, but cannot react with AgNPs, is also employed for the contrast experiments. The cell array is cultured successfully onto a Petri dish by microcontact printing (μCP) technique, which can provide a basic platform for studying of single cells. The approach curve and line scan are the two methods of SECM employed here to study the Hela cells. The former can provide the information about the interaction between Hela cells and AgNPs whereas the later gives the cell imaging. The permeability of cell membranes and morphology are two main factors which have effects on the feedback mode signals when K3Fe(CN)6 is used as the mediator. The permeability of the cell membranes can be ignored after interaction with high concentration of AgNP solution and the height of the Hela cells is slightly decreased in this process. The kinetic rate constants (k0) between IrCl62− and Ag on the Hela cell can be evaluated using K3IrCl6 as the mediator, and they are increased with the higher concentrations of the AgNP solutions. The k0 is changed about 10 times from 0.43 ± 0.04 × 10−4 to 1.25 ± 0.07 × 10−4 and to 3.93 ± 1.9 × 10−4 cm s−1 corresponding to 0, 1 and 5 mM of AgNO3 solution. The experimental results demonstrate that the AgNPs can be adsorbed on the cell surface and detected by SECM. Thus, the amount of AgNPs adsorbed on cell membranes and the permeability or morphology changes can be investigated simultaneously using this approach. The dual mediator system and cell array fabricated by μCP technique can provide better reproducibility because they can simplify experiments, and provide a platform for further single cell detection.
Introduction
Scanning electrochemical microscopy (SECM), an in situ technique, which has allowed one to study different types of interfaces and surfaces electrochemically with high spatial and temporal resolution, has previously been used to investigate biological systems.1–4 In the initial SECM investigations of biological samples by Bard et al. in 1990, the topographic images of surfaces of grass and Ligustrum sinensis leaves were obtained and the photosynthesis process was evaluated by the reduction of oxygen produced from an Elodea leaf.5 After that, the SECM has been increasingly employed for studying the living biological systems, and attempts have been recently undertaken in several aspects. For example, Matsue and coworkers have studied the metabolism, respiration and photosynthesis activities of a variety of individual living cells using generation-collection mode.6–12 Mirkin et al. have measured the intracellular redox activity and topography of different mammalian cell lines with hydrophobic mediators, which can diffuse across the cell membrane and react with the redox center in the cell using feedback mode of SECM.13,14 Schuhmann et al. have detected nitric oxide (NO) released from growing endothelial cell monolayers upon stimulation with a chemically modified NO-sensing microelectrode as the tip of SECM.15–17 Recently, Bard and coworkers have monitored and detected the export of thiodione, which was produced by the conjugation of glutathione and cytotoxic menadione in the living cells.18,19 They also reported that a liquid/liquid interface-based micropipette sensor could be used to monitor the toxicity of Ag+ to fibroblast cells.20 Jin's group has developed an electrochemical method for determination of enzyme activity inside single cells quantitatively by using a nanolitre-scale microcell.21,22 The combination of SECM and microbial array chips was also applied for detecting recombinant proteins23 and metabolic alteration in bacteria.24 Besides those studies, the permeability of the cell membranes to several types of redox couples was monitored by the feedback mode of SECM. Negative feedback signals were usually obtained with hydrophilic mediators (e.g., ferrocyanide or ferrocenecarboxylate), which could not pass through the cell membrane. In contrast, hydrophobic mediators (e.g., quinones such as menadione and 1,2-naphthoquinone) could diffuse across the cell membrane and react with redox moieties inside the cell, resulting in positive feedback signals.25 The tip current can be used to determine the effective kinetic rate constant of the overall process. Local permeability of the nuclear envelope at large intact nuclei isolated from Xenopus laevisoocytes was also studied by SECM.26 All those studies demonstrate that SECM is a promising technique for the investigation of living single cells.It is usually difficult to study cells or bacteria quantitatively at the single-cell level by SECM when the diameter of the SECM tip is comparable with the size of an individual cell.20 To make further progress in the development of the SECM based cellular sensing systems, it will be undoubtedly required to place cells in predetermined locations with defined shapes and sizes.27 In this way, it is easier to eliminate the effect of other cells around or near the cell under studied, and obtain more reliable information. Another approach is to employ a much smaller size of SECM tip, for instance, Mirkin et al. reported recently the electrochemical behavior of mammalian cells by SECM with nanoelectrodes as the tip.28 Microfabrication combined with surface chemistry and material science has provided a new possibility for further investigating the interactions of anchorage-dependent cells with their environments.29 A versatile ensemble of methods was developed during the last decade. For example, micro-contact printing (μCP), a soft lithography technique, is commonly used to create chemical structures on surfaces for controlling cell-substrate interactions. This method uses an elastic stamp with micrometre-scale features, which made from poly(dimethylsiloxane) (PDMS), for making monolayer patterns of blocking effect to the adsorption of extracellular matrix (ECM) proteins. Chilkoti and coworkers have reported a simple and generic method to micro-patterning surfaces with an amphiphilic comb polymer presenting short oligoethylene glycol side chains, which can prevent the non-specific adsorption of ECM proteins during cell culture.30 Single cell arrays can be easily obtained using such an approach through variation of the size of pattern.
The understanding of interactions between metal nanoparticles and living systems is of fundamental and practical interest due to metal particles in the nanometre size range that exhibit unique physical and chemical properties, different from both the ion and the bulk material themselves. Although the silver ion has long been known to have strong toxicity to a wide range of micro-organisms,31 the cytotoxicity of AgNPs has only been studied recently owing to the demands of the application of nanotechnology to biosystems. The practical application of the analysis of nucleolar organizer regions (NORs) in tumor pathology began in 1986, when a simple argyrophilic technique (silver-stained nucleolar organizer regions, AgNOR) to detect proteins associated with NORs was described.32 At present, the evaluation of AgNOR quantity can be regarded as an indicator of prognosis.33
The interactions between the AgNPs and the bacteria or cancer cells could be partially identified in three ways:33–35 (1) attaching to the surface of the cell membrane and disturbing its proper function, like permeability; (2) penetrating into the cell and causing further damage by possibly interacting with sulfur- and phosphorus-containing compounds such as DNA or NORs; (3) releasing silver ions, which will have an additional contribution to the bactericidal effect of the AgNPs. In previous work, scanning electron microscopy (SEM) was a common tool, which can be employed to investigate the change of cell morphologies under the attachment of AgNPs on the surface.36 However, it is hard to obtain the further information like the permeability of the cell membranes by this way. Girault's group has shown that the silver stained proteins adsorbed on poly(vinylidene difluoride) (PVDF) membranes can be imaged by SECM and developed a readout tool for detection of proteins with high sensitivity.37,38 This method actually provides a basis for the current studying of the interaction between cells and AgNPs by SECM.
In this work, we have tried to study the interaction between AgNPs and Hela cells by SECM with both K3Fe(CN)6 and K3IrCl6 as mediators in the same solution. A cell-resistant polymer, an amphiphilic comb polymer, was patterned onto a Petri dish using the μCP method, and then the Hela cell array could be obtained when culturing the Hela cells in proper experimental conditions. IrCl62−/3−, which can react with AgNPs, serves as the mediator to study the effect of the amount of AgNPs adsorbed on the cell membranes on the kinetic rate constants, whereas Fe(CN)63−/4−, which cannot react with AgNPs, has been used as the mediator to study the varying of the permeability or morphology of cell membranes when adding AgNP solution to the cell array. We have demonstrated that both mediators can be used simultaneously and have no interaction with each other during the detection. This will benefit the investigation because one does not need to change the solution in the process, which will make the results more compatible and reproducible.
Experimental
Chemicals and materials
All chemicals were used as received. Potassium hexachloroiridate(III) (K3IrCl6, Aldrich); potassium ferricyanide(III) (K3Fe(CN)6), sodium nitrate (NaNO3, >99%), silver nitrate (AgNO3, >99.5%), methanol and sodium borohydride (all supplied by Beijing Chemicals Co., Beijing, China). PVDF membranes (0.2 μm, Millipore). All aqueous solutions were prepared from the Millipore water (Milli-Q, Millipore Corp.).Comb polymers were synthesized according to the previously reported procedures, consisting of methyl methacrylate (MMA), poly(ethylene glycol methyl ether methacrylate) (referred herein as hydroxyl-poly(oxyethylene methacrylate) (HPOEM)), and poly(oxyethylene methacrylate) (POEM) via free-radical polymerization with a range of compositions.39
Synthesis of AgNPs
AgNPs were prepared by reduction of AgNO3 using NaBH4. In a 250 mL three-neck flask, 100 mL of AgNO3 (the concentration was 1 mM or 5 mM) was stirred with the addition of a little excess amount of NaBH4. Within very short time, the color changed from colorless to brown or black (the color of the nanoparticles solution changed with the concentrations of AgNO3). The prepared nanoparticles were characterized with transmission electron microscopy (TEM) (JEM 200CX, JEOL), and the size was determined to be 30–50 nm. They are easily to be coagulated without adding protected compound which will decrease the activity of the nanoparticles, therefore the AgNPs were freshly prepared before used. It is not easily to determine the concentration of AgNP solution so we use the concentration of AgNO3 solution (low: 1 mM; high: 5 mM) instead.Preparation of silver clusters on the PVDF membrane
The PVDF is hydrophobic, and it is necessary to be first wetted in methanol for 1–3 s and then immersed in water for 1–2 min to elute the methanol.37,38 Once the membrane has been wetted with water, do not allow it to be dry until the AgNPs adsorbed on it. The AgNP solution was spotted on the membranes by a microsyringe, and the diameters of the spots were about 1 mm. After evaporation of the solution, the AgNPs spot was slight black and the membrane can be characterized by SECM. The mediators were 1 mM K3Fe(CN)6 and 1 mM K3IrCl6 with 150 mM NaNO3 as the supporting electrolyte, and its concentration is similar to the commonly used physiological saline solution. This complex solution was also be used to study Hela cells in the following step.Cell culture and SECM measurements
The cell array was prepared after comb polymer was micropatterned using an oxidized poly(dimethylsiloxane) (PDMS) stamp which can provide micrometre-sized negative features based on the method reported previously.30 Briefly, the PDMS molds were fabricated by casting a polymer precursor (Sylgard 184 from Dow Corning) against a photoresist master that was fabricated by photolithography, then the viscous base and the curing agent (10 : 1 ratio by weight) were mixed well and cured at 80 °C for 2 h. The resulting PDMS stamp was 0.5 cm thick and cut into 1 cm × 1 cm pieces for further application. The stamp was immersed in a 1% (w/v) solution of the comb polymer in a 50 : 50 (v/v) H2O/ethanol mixture and brought into conformal contact with the bottom of the culture dish, resulting in the transfer of the comb polymer to the regions of the surface that were in contact with the stamp.The Hela cells were grown in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 units mL−1penicillin, 100 μg mL−1streptomycin, at 37 °C in 5% CO2. The cells were incubated for 24 h, and after that we could obtain the cell array because the micropatterned comb polymer is cell-resistant.
A Nikon TE-2000 inverted microscope (Nikon Co., Japan) was used to observe the coverage and growth status of the Hela cells. SECM and cyclic voltammetric (CV) measurements were performed with a CHI 910B workstation (CH Instrument Co., Austin, TX) which mounted on the stage of the inverted microscope. The three-electrode system consisted of a 12.5 μm radius Pt working electrode, an Ag/AgCl electrode as the reference electrode, and a Pt wire as the auxiliary electrode.
Before the measurement, the physiological saline solution was replaced by 150 mM NaNO3, and then added the newly synthesized AgNP solution. The interaction of AgNPs and cells was limited to 10 min, and then the cells were washed using 150 mM NaNO3 solution twice carefully to repulse the excessive silver nanoparticles in the solution, and detected by SECM in 150 mM NaNO3 solution with 1 mM K3IrCl6 and 1 mM K3Fe(CN)6 as the mediators.
Results and discussion
Characterization of silver clusters on the PVDF membrane by SECM
In order to test the dual-mediator system, we have chosen the well studied PVDF membrane as a model system for living cells. Based on the previous work,37,38,40 we know that there are two possible mechanisms for the signal generation between the SECM tip and AgNPs. One is the conventional feedback mode which depends upon the inherent sensitivity of SECM to the detection of small variation of the surface conductivities, as described in ref. 40. In this case, it requires a large amount of interconnected silver clusters to provide a conductive substrate. The other is an etching process of AgNPs induced by the SECM tip, which relies on a bimolecular electron transfer (ET) reaction between an oxidation species formed at the tip and AgNPs deposited on a substrate (PVDF membrane or a living cell). In the dual-mediator system, K3Fe(CN)6 and K3IrCl6, based on these two mechanisms, respectively, are chosen as the mediators to detect the silver clusters deposited on PVDF membranes, which can be used as the model for study of the interaction between cells and AgNPs. The cyclic voltammograms shown in Fig. 1 are observed on a 25 μm diameter Pt electrode with (solid curve) or without the redox couples (dash line). The two redox couples are having almost the same steady-state current because they have the same concentrations (1 mM for each other), similar diffusion coefficients (7.6 × 10−6 cm2 s−1 for K3Fe(CN)6 and 8.2 × 10−6 cm2 s−1 for K3IrCl641) and the same charge involved in the redox processes in the system with 150 mM NaNO3 as the supporting electrolyte. These CV curves indicate that the half-wave potentials on the tip are about 0.05 V for K3Fe(CN)6, and 0.65 V for K3IrCl6 (vs.Ag/AgCl). More importantly, they do not interfere each other.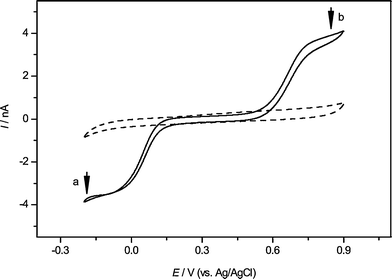 | ||
| Fig. 1 The cyclic voltammograms obtained by the Pt microelectrode in the 150 mM NaNO3 solution as the blank (dash line) or including 1 mM K3IrCl6 (b) and 1 mM K3Fe(CN)6 (a) (solid line) as the mediators. The scan rate was 10 mV s−1, and Ag/AgCl served as the reference electrode. The arrows indicate the tip voltage for each mediator during the SECM experiments. | ||
Using the same solution containing K3Fe(CN)6 and K3IrCl6, two series of data for SECM lateral scans over the same position with each of them as mediator have been obtained (see the ESI‡). The signals based on K3IrCl6 are about five times larger than those based on K3Fe(CN)6. This is because the Fe(CN)64− (generated by the tip reaction) cannot react with the AgNPs, which only act as an insulating substrate, while IrCl62−, which has a more positive standard potential, can oxidize the AgNPs into Ag+ and cause the enhancement of the SECM feedback signals:
| Tip: IrCl63− − e → IrCl62− | (1) |
| Substrate: Ag(s) + IrCl62− → Ag+(l) + IrCl63− | (2) |
Girault et al. have demonstrated that SECM can be employed as a sensitive and quantitative readout method for the detection of proteins adsorbed on PDVF membranes after stained by AgNPs, based on similar reactions.37
Approach curve above a single living Hela cell
The combination of microfabrication and scanning probe techniques is useful for studies of single cells. From the diagram shown in Fig. 2A obtained by optical microscopy, it is clearly that the cell array was cultured successfully onto a Petri dish. The pattern can be varied according to our purpose. In the present experiments, the size of the “cell island” is about 20 μm in diameter, and the distance between two nearest “cell islands” is about 30 μm. Almost all the cells will be separated each other, but there are still some cells striding over two “islands”, which will be very interesting to be studied in the future. There are also some blank islands without cells, which is rather common when the cells’ density is not high enough. In order to investigate the interaction between AgNPs and Hela cell, the newly prepared AgNPs should be pre-mixed with the cell array, and then incubated at room temperature for 10 min. Fig. 2B is the TEM diagram of the freshly prepared AgNPs with a diameter about 30–50 nm.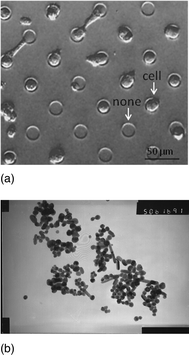 | ||
| Fig. 2 (a) The cell array cultured onto the Petri dish, pictured by the inverted microscopy. The diameter of cell island is about 20 μm and the distance between two nearest islands is about 30 μm. (b) The TEM image of AgNPs, and the diameters of them were between 30–50 nm. | ||
After finding a Hela cell and positioning the SECM tip above it, with the help of the optical microscope, the current–distance (approach) curves can be obtained, which are shown in Fig. 3, and the relevant kinetic rate constants can be obtained by fitting the experimental curves to the theoretical ones (see Table 1). Fig. 3A shows the approach curve for the case when K3IrCl6 is used as the mediator. Positive feedback is observed when approaching the AgNP clusters adsorbed on PVDF membrane, which is consistent with the line scan results in the previous section (see ESI‡). The effective heterogeneous kinetic rate constants (k0) of reactions between K3IrCl6 and different substrates are evaluated and listed in Table 1. The rate constant for the cell without AgNPs is similar to that of the PVDF as the substrate, and negative feedback is obtained. Fig. 3B shows the case when K3Fe(CN)6 is used as the mediator. All the feedback curves are almost overlapping each other, which indicates that the reaction between the mediator and cell or AgNPs is very slow.
| Substrate | Rate constant (k0) × 10−4/cm s−1 | |
|---|---|---|
| Mediator (1 mM) | ||
| K3IrCl6 | K3Fe(CN)6 | |
| PVDF | 0.45 ± 0.07 | 0.45 ± 0.05 |
| PVDF + AgNPs | 50.0 ± 3.0 | 0.85 ± 0.07 |
| Cell | 0.43 ± 0.04 | 0.45 ± 0.07 |
| Cell + AgNPs (low concentration) | 1.25 ± 0.07 | — |
| Cell + AgNPs (high concentration) | 3.93 ± 1.9 | 0.55 ± 0.06 |
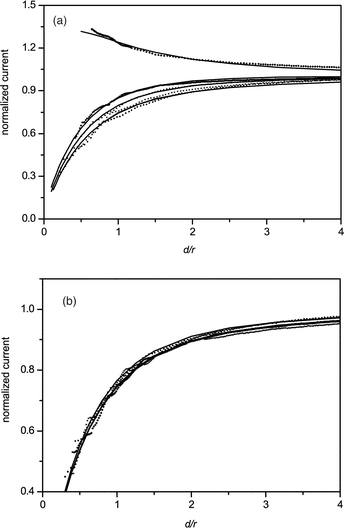 | ||
| Fig. 3 Normalized approach curves. Five curves are shown in (A) using K3IrCl6 as the redox mediator, from top to the bottom are: (1) PVDF + AgNPs, (2) Hela cell + AgNPs (high concentration), (3) Hela cell + AgNPs (low concentration), (4) Hela cell, (5) PVDF. Four curves are shown in (B) using K3Fe(CN)6 as the redox mediator, from top to the bottom are (1) PVDF + AgNPs, (2) Hela cell + AgNPs (large concentration), (3) Hela cell, (4) PVDF. The tip potential was held at 0.65 V for K3IrCl6 and 0.05 V for K3Fe(CN)6 (vs.Ag/AgCl). The approach rates are 10 μm s−1. | ||
According to our results and previous reports, there are at least three factors influencing the feedback signals in SECM experiments using Fe(CN)64− and IrCl62− as redox mediators for the same cell array: (1) the reaction between the mediators and AgNPs adsorbed on the cell membrane, (2) the permeability of the cell membrane for the mediators, (3) the reaction between the mediators and intracellular redox species (see Fig. 4). The permeability is depended mainly on the hydrophilicity of mediator and the perforation in cell membrane after interaction with AgNPs. Since the hydrophilicity is similar for Fe(CN)64− and IrCl62−, and the interaction process between cell and AgNPs is identical in our experiments, so the permeability for the two mediators should be similar. From Fig. 3B and Table 1, the rate constants for the Hela cell before and after the interaction with AgNPs have no significant difference when K3Fe(CN)6 is used as the mediator. Therefore, the change of permeability of the cell membrane can be ignored in this process and there is no perforation in these concentrations of AgNPs. It has been reported that after treatment with AgNPs, perforation appears in the bacteria cell membranes,36 which will change the cell permeability, but the constitution and configuration of the membranes in bacteria and cancer cells are quite different from each other, so that the perforation may not appear in the current studies. That is, such hydrophilic mediators will still hardly be able to pass through the cell membrane and react with the intracellular redox species. Hence the signal differences between the two mediators are mainly attributed to the interactions between the SECM tip and AgNPs deposited on the Hela cells or species on the cells’ surfaces, and processes 2 and 3 shown in Fig. 4 can be ignored in the present work. After interaction with AgNPs, the Hela cell shows a larger rate constant using K3IrCl6 as mediator, but still smaller than AgNPs clusters adsorbed on PVDF as the substrate. This is probably because the density of AgNPs adsorbed on the cell membrane is smaller than that adsorbed on the PVDF. The cell after interaction with a higher concentration of AgNP solution shows a higher rate constant than the one with a smaller concentration, which means the amount of AgNP adsorbed on the cell membranes is increased with the higher concentration of AgNP solution. These experimental results demonstrate that we can investigate simultaneously the permeability of cell membranes using K3Fe(CN)6 as the mediator and the effect of the amount of AgNPs adsorbed on the Hela cell membranes using K3IrCl6 as the mediator.
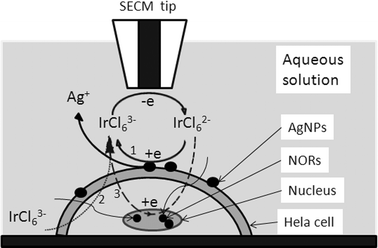 | ||
| Fig. 4 The operating principle of SECM to detect the interaction between AgNPs and Hela cells. When the tip approaches the cell, the IrCl63− is regenerated in three ways: (1) IrCl62− reduced by the AgNPs adsorbed on the cell membranes; (2) IrCl63−, which is plentiful in the bulk solution, may penetrate into cells and diffuse away from the tip; (3) IrCl62− penetrates into the cells and is reduced by intracellular redox species or the AgNPs in the NORs. | ||
Imaging the cell array
An approach curve was taken to control the distance between the tip and the bottom of the Petri dish over a region without cells. The tip is stopped when the current is 30% of the steady-state current, which is about 3 μm from the Petri dish according to the theoretical calculation.1–3 Then the SECM tip is withdrawn 15 μm to avoid touching of the cells. Thus, the tip is moved to the cell array to perform the probe scanning laterally experiments with the help of the inverted microscopy. The interaction process between Hela cells and AgNPs is similar to that previously described. The negative feedback currents of the two mediators are almost the same (see Fig. 5A) in the absence of AgNPs, indicating the cells only act as obstacles when there are no AgNPs on the cell membranes, and there is no communication between the cells and the SECM tip, or it is too small to be detected by this method. While in Fig. 5B, the negative feedback currents of K3IrCl6 are obviously smaller than those of K3Fe(CN)6 in the presence of AgNPs, which is partially because of the adsorption of the AgNPs on the cell membranes. The negative feedback signals of K3IrCl6 are decreased with the increasing of AgNP concentrations and the positive feedback signals are observed ultimately (Fig. 5C). These results demonstrate that the amount of the AgNPs deposited on the cell membranes depends on the AgNP concentration in the solution, which is consistent with the results obtained in the previous section. According to the Brønsted acid–base theory, silver will tend to have a higher affinity to react with phosphorus and sulfur compounds, which are plentiful in cell membrane proteins,35 therefore the AgNPs can be deposited on the Hela cell membranes randomly.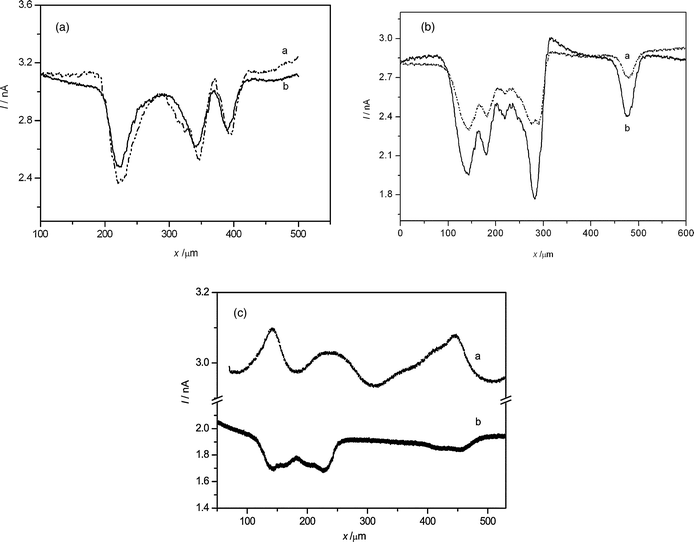 | ||
| Fig. 5 Feedback currents for the lateral scanning of the Hela cell arrays using (a) K3IrCl6 and (b) K3Fe(CN)6 in 150 mM NaNO3. There were no silver nanoparticles (A) or different concentrations (B: low, C: 10 times greater than that of B) of AgNPs interacting with the Hela cells. The scan rate was 30 μm s−1. | ||
Although Fe(CN)64− cannot oxidize the silver as we have demonstrated in the previous sections, the negative feedback currents using K3Fe(CN)6 as a mediator are a little decreased with the increasing of the concentration of AgNP (the ratios of peak current and background current are 0.78 ± 0.07 for Fig. 5B and 0.90 ± 0.04 for Fig. 5C, see Table 2). The permeability of cell membranes or the heights of the cell are two factors which will influence the feedback signals during a constant height scan above the cell arrays. A similar method was used by Baur et al.42,43 to study the morphology of PC12 cells. In the previous section, we demonstrated that the permeability has little change after interaction with AgNPs, so this difference is probably due to the height of the Hela cells being decreased at higher concentrations of AgNPs.
| Current ratio | Mediators (1 mM) | |
|---|---|---|
| K3IrCl6 | K3Fe(CN)6 | |
| A | 0.81 ± 0.06 | 0.85 ± 0.05 |
| B | 0.87 ± 0.06 | 0.78 ± 0.07 |
| C | 1.0 ± 0.02 | 0.90 ± 0.04 |
SECM can also be employed for electrochemical mapping of redox activity of individual living cells. Constant height and constant distance are the two commonly used modes for the SECM imaging. Schuhmann and coworkers have introduced a shear-force based constant-distance approach to control the SECM tip precisely,44,45 but the constant height mode is technically simple and easy to be used. Fig. 6A and C show images of the cell array obtained by the SECM constant height mode. The redox activity maps obtained by SECM are compared with the optical micrographs captured for the same fields (Fig. 6B and D). Pure negative feedback imaging of the Hela cell array without adding AgNPs can be obtained in the presence of K3IrCl6. On the contrary, positive feedback imaging can be also obtained after the interaction with higher concentration of AgNPs and cells. These phenomena are consistent with the lateral scan of the cell array. We noticed that the sizes of cells imaged by SECM were slightly larger than those obtained by the optical method. This is probably because the electrochemical signals of the mediators detected by the tip were in a diffusion field, so that the results were larger than the real size. After the interaction with AgNPs with high density, the activity of cells decreased and some of them were easy to glide from “cell islands” indicating by the red circles in Fig. 6C. This may also be the damage caused by the addition of AgNPs. It is hard to obtain higher spatial resolution with the present micro-sized SECM tip. A study of using a nanometre-sized SECM tip to analyse the local electrochemical reactivities is being undertaken in our laboratory.
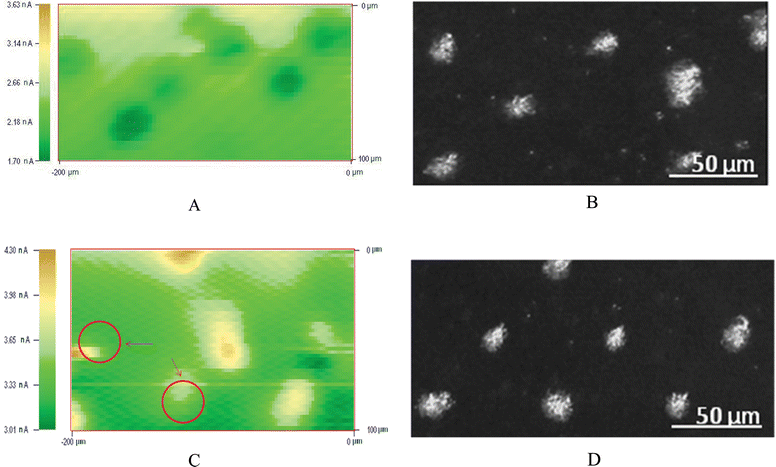 | ||
| Fig. 6 Optical micrographs (B and D) and SECM images (A and C, 200 μm × 100 μm) of island patterns of Hela cells formed onto the Petri dish. The red circles in C indicate the lateral excursion of the cells after the interaction with AgNPs, which stands for the decreasing of the activities. | ||
Conclusions
The interactions between Hela cells and AgNPs have been investigated by SECM with the help of dual electro-active mediators as probing species. Fabrication of the cell array simplifies the study at single cell level and provides a platform for further investigations of interactions of AgNPs and other chemical substances, for instance, drugs. The variation of permeability of Hela cell membranes after the interaction with AgNPs can be ignored because all the approach curves show little change under the concentration range of AgNPs studied in this work when Fe(CN)63−/4− is used as the mediator, and the height of the cell is slightly decreased in this process. The approach curves are influenced by the AgNPs deposited on the Hela cell, and the rate constants are increased with the increase of concentration of AgNPs in solution when K3IrCl6 is used as the mediator. This is due to IrCl62− produced in situ, which can oxidize the AgNPs. A more detailed study about quantitative analysis of the interaction between AgNPs and the Hela cells, and the interaction mechanism are being undertaken in our laboratory. Apparently, a much smaller size of SECM tip is necessary.Acknowledgements
This work was supported by the National Natural Science Foundation of China (20735001, 20628506), the Foundation of Doctoral Programs of the Ministry of Education of China, and the special 985 project of Peking University.References
- B. R. Horrocks and G. Wittstock, Biological systems, in Scanning Electrochemical Microscopy, ed. A. J. Bard and M. V. Mirkin, Marcel Dekker, New York, 2001, p. 445 Search PubMed.
- S. Amemiya, J. Guo, H. Xiong and D. A. Gross, Anal. Bioanal. Chem., 2006, 386, 458 CrossRef CAS.
- A. J. Bard, X. Li and W. Zhan, Biosens. Bioelectron., 2006, 22, 461 CrossRef CAS.
- M. A. Edwards, S. Martin, A. L. Whitworth, J. V. Macpherson and P. R. Unwin, Physiol. Meas., 2006, 27, R63 CrossRef.
- C. Lee, J. Kwak and A. J. Bard, Proc. Natl. Acad. Sci. U. S. A., 1990, 87, 1740 CAS.
- H. Shiku, T. Shiraishi, H. Ohya, T. Matsue, H. Abe, H. Hoshi and M. Kobayashi, Anal. Chem., 2001, 73, 3751 CrossRef CAS.
- Y. Torisawa, T Kaya, Y. Takii, D. Oyamatsu, M. Nishizawa and T. Matsue, Anal. Chem., 2003, 75, 2154 CrossRef CAS.
- T. Kaya, Y.-S. Torisawa, D. Oyamatsu, M. Nishizawa and T. Matsue, Biosens. Bioelectron., 2003, 18, 1379 CrossRef CAS.
- Y. Torisawa, H. Shiku, S. Kasai, M. Nishizawa and T. Matsue, Int. J. Cancer, 2004, 109, 302 CrossRef CAS.
- H. Shiku, Y.-S. Torisawa, A. Takagi, S. Aoyagi, H. Abe, H. Hoshid, T. Yasukawa and T. Matsue, Sens. Actuators, B, 2005, 108, 597 CrossRef.
- H. Shiku, Y.-S. Torisawa, A. Takagi, S. Aoyagi, H. Abe, H. Hoshid, T. Yasukawa and T. Matsue, Sens. Actuators, B, 2005, 108, 597 CrossRef.
- T. Saito, C. Wu, H. Shiku, T. Yasukawa, M. Yokoo, T. Ito-Sasaki, H. Abe, H. Hoshid and T. Matsue, Analyst, 2006, 131, 1006 RSC.
- B. Liu, S. A. Rotenberg and M. V. Mirkin, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 9855 CrossRef CAS.
- B. Liu, S. A. Rotenberg and M. V. Mirkin, Anal. Chem., 2002, 74, 6340 CrossRef CAS.
- A. Pailleret, J. Oni, S. Reiter, S. Isik, M. Etienne, F. Bedioui and W. Schuhmann, Electrochem. Commun., 2003, 5, 847 CrossRef CAS.
- S. Isik, M. Etienne, J. Oni, A. Blöchl, S. Reiter and W. Schuhmann, Anal. Chem., 2004, 76, 6389 CrossRef CAS.
- S. Borgmann, I. Radtke, T. Erichsen, A. Blöchl, R. Heumann and W. Schuhmann, ChemBioChem, 2006, 7, 662 CrossRef CAS.
- J. Mauzeroll and A. J. Bard, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 7862 CrossRef CAS.
- J. Mauzeroll, A. J. Bard, O. Owhadian and T. J. Monks, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 17582 CrossRef CAS.
- D. Zhan, X. Li, W. Zhan, F.-R. F. Fan and A. J. Bard, Anal. Chem., 2007, 79, 5225 CrossRef CAS.
- N. Gao, M. Zhao, X. Zhang and W. Jin, Anal. Chem., 2006, 78, 231 CrossRef CAS.
- X. Zhang, F. Sun, X. Peng and W. Jin, Anal. Chem., 2007, 79, 1256 CrossRef CAS.
- K. Nagamine, S. Onodera, A. Kurihara, T. Yasukawa, H. Shiku, R. Asano, I. Kumagai and T. Matsue, Biotechnol. Bioeng., 2007, 96, 1008 CrossRef CAS.
- K. Nagamine, N. Matsui, T. Kaya, T. Yasukawa, H. Shiku, T. Nakayama, T. Nishino and T. Matsue, Biosens. Bioelectron., 2005, 21, 145 CrossRef CAS.
- B. Liu, S. A. Rotenberg and M. V. Mirkin, Anal. Chem., 2002, 74, 6340 CrossRef CAS.
- J. Guo and S. Amemiya, Anal. Chem., 2005, 77, 2147 CrossRef CAS.
- M. Nishizawa, K. Takoh and T. Matsue, Langmuir, 2002, 18, 3645 CrossRef.
- P. Sun, F. O. Laforge, T. P. Abeyweera, S. A. Rotenberg, J. Carpino and M. V. Mirkin, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 443 CrossRef CAS.
- D. Falconnet, G. Csucs, H. M. Grandin and M. Textor, Biomaterials, 2006, 27, 3044 CrossRef CAS.
- J. Hyun, H. Ma, Z. Zhang, T. P. Jr. Beebe and A. Chilkoti, Adv. Mater., 2003, 15, 576 CrossRef CAS.
- K. B. Holt and A. J. Bard, Biochemistry, 2005, 44, 13214 CrossRef CAS.
- D. Ploton, M. Menager, P. Jeannesson, G. Himber, F. Pigeon and J. J. Adnet, Histochem. J., 1986, 18, 5 CrossRef CAS.
- A. Pich, L. Chiusa and E. Margaria, Micron, 2000, 31, 133 CrossRef CAS.
- X.-H. N. Xu, W. J. Brownlow, S. V. Kyriacou, Q. Wan and J. J. Viola, Biochemistry, 2004, 43, 10400 CrossRef CAS.
- J. R. Morones, J. L. Elechiguerra, A. Camacho, K. Holt, J. B. Kouri, J. T. Ramírez and M. J. Yacaman, Nanotechnology, 2005, 16, 2346 CrossRef CAS.
- S. K. Gogoi, P. Gopinath, A. Paul, A. Ramesh, S. S. Ghosh and A. Chattopadhyay, Langmuir, 2006, 22, 9322 CrossRef CAS.
- M. Zhang, G. Wittstock, Y. Shao and H. H. Girault, Anal. Chem., 2007, 79, 4833 CrossRef CAS.
- M. Zhang and H. H. Girault, Electrochem. Commun., 2007, 9, 1778 CrossRef CAS.
- H. Ma, J. Hyun, Z. Zhang, T. P. Jr. Beebe and A. Chilkoti, Adv. Funct. Mater., 2005, 15, 529 CrossRef CAS.
- J. Wang, F. Y. Song and F. M. Zhou, Langmuir, 2002, 18, 6653 CrossRef CAS.
- J. F. Llopis and F. Colom, in Encyclopedia of Electrochemistry of the Elements, ed. A. J. Bard, Marcel Dekker, New York, 1976, vol. 6, p. 224 Search PubMed.
- J. M. Liebetrau, H. M. Miller, J. E. Baur, S. A. Takacs, V. Anupunpisit, P. A. Garris and D. O. Wipf, Anal. Chem., 2003, 75, 563 CrossRef.
- R. T. Kurulugama, D. O. Wipf, S. A. Takacs, S. Pongmayteegul, P. A. Garris and J. E. Baur, Anal. Chem., 2005, 77, 1111 CrossRef CAS.
- L. P. Bauermann, W. Schuhmann and A. Schulte, Phys. Chem. Chem. Phys., 2004, 6, 4003 RSC.
- A. Hengstenberg, C. Kranz and W. Schuhmann, Chem.–Eur. J., 2000, 6, 1547 CrossRef CAS.
Footnotes |
| † This paper is part of an Analyst themed issue highlighting Chinese science, with guest editor Mengsu (Michael) Yang. |
| ‡ Electronic supplementary information (ESI) available: Fig. S1, experiment for the lateral scanning over the silver nanoparticles spots with different mediators. See DOI: 10.1039/b807057a |
| This journal is © The Royal Society of Chemistry 2008 |
