Enhanced near-infrared emission by co-doping Ce3+ in Ba2Y(BO3)2Cl:Tb3+, Yb3+ phosphor
Jin Zhao,
Chongfeng Guo* and
Ting Li
National Key Laboratory of Photoelectric Technology and Functional Materials (Culture Base) in Shaanxi Province, National Photoelectric Technology and Functional Materials & Application of Science and Technology International Cooperation Base, Institute of Photonics & Photon-Technology, Northwest University, Xi’an 710069, China. E-mail: guocf@nwu.edu.cn; Fax: +86-29-88302661; Tel: +86-29-88302661
First published on 12th March 2015
Abstract
Ce3+–Tb3+–Yb3+ tri-doped Ba2Y(BO3)2Cl phosphors with intense near-infrared (NIR) emission and broad band absorption in the near ultraviolet region were synthesized by a convenient solid-state reaction in a reducing atmosphere, and the structure and the phase purity of all samples were characterized using X-ray diffraction (XRD). The visible and NIR emission spectra together with corresponding decay curves have also been measured to investigate the energy transfer (ET) processes and analysis ET mechanisms and efficiencies in Ce3+–Tb3+–Yb3+ tri-doped system. In addition, steady and dynamic luminescent properties of Tb3+/Ce3+–Yb3+ co-doped Ba2Y(BO3)2Cl phosphors were also measured as a comparison. As an excellent sensitizer, Ce3+ ion could significantly enlarge the absorption cross-section and greatly enhance the NIR emission intensity of Yb3+ ion. It is believed that Ce3+–Tb3+–Yb3+ tri-doped Ba2Y(BO3)2Cl phosphor is a promising down-conversion (DC) solar spectral convertor to enhance the efficiency of the silicon solar cells.
1. Introduction
Due to the global energy crisis, much attention has been paid to green and renewable energy. Solar cells are recognized as the most promising devices to convert solar energy to electricity, while silicon solar cells occupy a dominant position in the solar market.1–3 The major energy loss in crystalline Si (c-Si) solar cells is a spectral mismatch between the incident solar spectrum and the silicon spectral response, which is the main reason for the lower photoelectric conversion efficiency.4 The photons with lower energy than the band gap (Eg ≈ 1.12 eV, λ ≈ 1100 nm) of silicon are not absorbed, while the photons with much higher energy than the band gap lose their excess energy through thermalization of hot charge carriers.5 A promising method to reduce the thermalization effect is to turn one ultraviolet-visible (UV-Vis) high energy photon into one or more available near infrared (NIR) low energy photons via down-conversion (DC) luminescent materials, including down shift (DS, shift short-wavelength light to long-wavelength light) and quantum cutting (QC, split one incident high energy photon into two near infrared photons) processes. Since efforts are being made to enhance the efficiency of silicon solar cells, the highest efficiency is not limited to 30% (the Shockley–Queisser limit) via spectral modification with DC phosphors.6,7Recently, RE3+–Yb3+ (RE = Tb3+, Pr3+, Tm3+, Ho3+ or Er3+) ion pairs co-doped QC materials have been extensively investigated, in which Yb3+ ion is a suitable emitter and acceptor with its emission around 1000 nm NIR and matches well with the band gap of c-Si due to its simple energy levels of 2F5/2 and 2F7/2 (separated by ∼10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1).8 According to the quantum cutting concept, one blue photon absorbed by RE3+ due to the absorption of Tb3+: 7F6 → 5D4 around 486 nm, Pr3+: 3H4 → 3P2 around 445 nm, Ho3+: 5F3 → 5I8 around 447 nm and so on could be divided into two NIR photons of Yb3+ ion by the 2F5/2 → 2F7/2 transition, which may lower the adverse thermalization effect caused by excess energy.9 However, these RE3+ ions suffer from narrow absorption cross sections (in the order of 10−21 cm2) and low absorption intensity due to the forbidden 4f–4f transitions. Thus, the NIR emission from Yb3+ is so weak that application of RE3+–Yb3+ (RE = Tb3+, Pr3+, Tm3+, Ho3+ or Er3+) co-doped QC materials in silicon solar cells is limited.10–13 Unlike other RE3+ ions, Ce3+ ions with allowed 4f–5d transitions generally have broad and strong absorption (in the order of 10−18 cm2) in the UV-Vis region.14 Ce3+ ion could be used as an efficient sensitizer and transfer its energy absorbed from the UV-Vis region to Tb3+ or Yb3+ effectively.15 In addition, cooperative energy transfer (CET) mechanisms of RE3+ → 2Yb3+ (e.g. RE = Ce3+, Tb3+, Pr3+, Tm3+) were theoretically realizable and had been investigated in detail, which is a possible way of energy transfer (ET) in RE3+–Yb3+ pair.16,17 According to our previous result, phosphor Ba2Y(BO3)2Cl:Ce3+, Tb3+ exhibited both a blue emission from Ce3+ and a green emission from Tb3+ under near ultraviolet light excitation in the range 320–390 nm, energy transfer from Ce3+ to Tb3+ was efficient.18 Therefore, the energy transfer from Ce3+ to Tb3+–Yb3+ ion pairs can occur.
000 cm−1).8 According to the quantum cutting concept, one blue photon absorbed by RE3+ due to the absorption of Tb3+: 7F6 → 5D4 around 486 nm, Pr3+: 3H4 → 3P2 around 445 nm, Ho3+: 5F3 → 5I8 around 447 nm and so on could be divided into two NIR photons of Yb3+ ion by the 2F5/2 → 2F7/2 transition, which may lower the adverse thermalization effect caused by excess energy.9 However, these RE3+ ions suffer from narrow absorption cross sections (in the order of 10−21 cm2) and low absorption intensity due to the forbidden 4f–4f transitions. Thus, the NIR emission from Yb3+ is so weak that application of RE3+–Yb3+ (RE = Tb3+, Pr3+, Tm3+, Ho3+ or Er3+) co-doped QC materials in silicon solar cells is limited.10–13 Unlike other RE3+ ions, Ce3+ ions with allowed 4f–5d transitions generally have broad and strong absorption (in the order of 10−18 cm2) in the UV-Vis region.14 Ce3+ ion could be used as an efficient sensitizer and transfer its energy absorbed from the UV-Vis region to Tb3+ or Yb3+ effectively.15 In addition, cooperative energy transfer (CET) mechanisms of RE3+ → 2Yb3+ (e.g. RE = Ce3+, Tb3+, Pr3+, Tm3+) were theoretically realizable and had been investigated in detail, which is a possible way of energy transfer (ET) in RE3+–Yb3+ pair.16,17 According to our previous result, phosphor Ba2Y(BO3)2Cl:Ce3+, Tb3+ exhibited both a blue emission from Ce3+ and a green emission from Tb3+ under near ultraviolet light excitation in the range 320–390 nm, energy transfer from Ce3+ to Tb3+ was efficient.18 Therefore, the energy transfer from Ce3+ to Tb3+–Yb3+ ion pairs can occur.
In this paper, we investigated Ce3+–Tb3+–Yb3+ tri-doped Ba2Y(BO3)2Cl phosphor. The visible and NIR emission spectra and corresponding decay curves were measured to prove the occurrence of ET in the present system, and the ET mechanisms were systematically studied and discussed. The results demonstrate that Ce3+ ion could efficiently sensitize the Tb3+–Yb3+ pair and enhance the NIR emission of Yb3+ ion greatly.
2. Experimental
All the Ba2Y(BO3)2Cl:Tb3+/Ce3+, Yb3+ and Ba2Y(BO3)2Cl:Ce3+, Tb3+, Yb3+ powder samples were prepared by a conventional solid-state reaction in a reducing atmosphere. Analytical grade BaCO3, BaCl2·2H2O, H3BO3 (excess 5 mol% to compensate for the evaporation), and high purity (99.99%) rare earth oxides Y2O3, Yb2O3, Tb4O7, CeO2 were used as raw materials. The composition for each sample was weighed in proper stoichiometric ratio and mixed thoroughly in an agate mortar. The mixture samples were preheated at 500 °C for 2 h in air. After being cooled to room temperature, the sample was thoroughly reground and heated for 4 h in a CO atmosphere at 900 °C to ensure the complete reduction of Ce3+ and Tb3+. It was then cooled to room temperature and crushed into a fine powder.The X-ray diffraction (XRD) patterns were performed on a Rigaku-Dmax 3C powder diffractometer (Rigaku Corp, Tokyo, Japan) with Cu-Kα radiation (λ = 1.54065 Å) to identify the structure and phase purity. The photoluminescence emission (PL) and excitation (PLE) spectra and decay curves were recorded using an Edinburgh FLS920 spectrofluorometer with a 450 W Xe lamp as excitation source. Decay curve measurements of Ce3+ ion were obtained using a 375 nm nanosecond flash-lamp (nF900) as the excitation source. Photoluminescent spectra of all samples were tested three times to reduce the error and all of the measurements were performed at room temperature.
3. Results and discussions
3.1 Phase and structure
Fig. 1 shows the X-ray diffraction (XRD) patterns of Tb3+ solely doped, Tb3+–Yb3+ co-doped and Ce3+–Tb3+–Yb3+ tri-doped Ba2Y(BO3)2Cl samples, respectively. The diffraction peaks of all samples agree well with those of the standard profile (JCPDS no. 79-0967) Ba2Yb(BO3)2Cl and not any diffraction peaks from impurity. It is noted that all samples show similar XRD patterns in our experiments, which indicate that the obtained samples are single and pure phases, and RE3+ (Ce3+/Tb3+/Yb3+) ions completely enter the lattices even at high concentrations. Here, most of the rare earth ion dopants would prefer to occupy a Y3+ site in the Ba2Y(BO3)2Cl compound according to the close effective ionic radius and valence of the cations. Compound Ba2Y(BO3)2Cl shares the same structure with Ba2Yb(BO3)2Cl, which crystallizes in a monoclinic structure with the space group P21/m, having the lattice parameters of a = 6.397 Å, b = 5.279 Å, c = 11.222 Å.19 | ||
| Fig. 1 XRD patterns of phosphors Ba2Y(BO3)2Cl:Tb3+, Ba2Y(BO3)2Cl:Tb3+, Yb3+ and Ba2Y(BO3)2Cl:Ce3+, Tb3+, Yb3+ along as well with the standard profile for Ba2Yb(BO3)2Cl (JCPDS card no. 79-0967). | ||
3.2 Analysis of photoluminescence spectra and energy transfer processes
Fig. 2b exhibits the PLE and PL spectra of Ba2Y(BO3)2Cl:0.03Ce3+, 0.20Yb3+ phosphor. Upon 355 nm excitation, the phosphor generates the blue emission from Ce3+ and NIR emission from Yb3+. For the PLE spectra monitoring both the 5d → 4f transition of Ce3+ at 417 nm and the 2F5/2 → 2F7/2 transition of Yb3+ at 972 nm, which are similar except for the intensity concluding an intense excitation band of Ce3+: 4f → 5d centered at 355 nm, and this indicates the energy transfer from Ce3+ to Yb3+.23 It is observed that the emission of Ce3+ decreases and the emission of Yb3+ increases with increasing the contents of Yb3+, whereas the emission intensity of Yb3+ reaches the maximum at x = 0.2 due to concentration quenching effect (as shown in Fig. 2d). Regular changes in the emission intensities from Ce3+ or Yb3+ also provide powerful evidence for an ET process from Ce3+ to Yb3+.
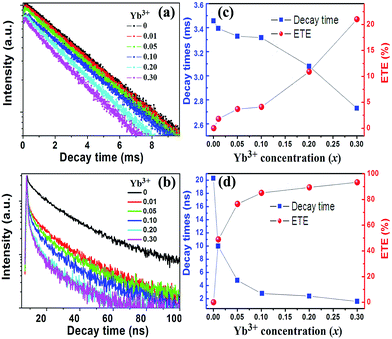
In order to further prove for the occurrence of the energy transfer from Tb3+/Ce3+ to Yb3+ and gain understanding of the ET process, the decay curves of the Tb3+: 5D4 → 7F5 emission at 542 nm in Ba2Y(BO3)2Cl:0.03Tb3+, xYb3+ and the Ce3+: 5d → 4f emission at 417 nm in Ba2Y(BO3)2Cl:0.03Ce3+, xYb3+ were recorded in Fig. 3a and b. The Tb3+ 542 nm emission without Yb3+ shows a nearly single exponential decay, whereas the Ce3+ 417 nm emission without Yb3+ deviates from single exponential decay because of the energy transfer between possible distribution of Ce3+ in different cation sites (two Ba2+ cites and one Y3+ site).14 For a sample without any Yb3+, the decay time of 3.46 ms and 20.31 ns are obtained for Tb3+ and Ce3+ by fitting. With the introduction of Yb3+ and increasing its concentration, their decay curves became nonexponential and the lifetime of the sensitizer Tb3+ or Ce3+ decreases. The average lifetime (τ) of sensitizer Tb3+/Ce3+ in Tb3+/Ce3+–Yb3+ co-doped Ba2Y(BO3)2Cl samples is given by:24
 | (1) |
Fig. 3c and d present the emission average lifetime and energy transfer efficiency of Tb3+/Ce3+–Yb3+ co-doped Ba2Y(BO3)2Cl samples as function of Yb3+ concentrations. According to the decay times, the energy transfer efficiency (ETE, ηETE) can be calculated by using the following equations:25
| ηETE = 1 − τxYb/τ0Yb | (2) |
The energy transfer process is complicated in Ce3+–Tb3+–Yb3+ tri-doped Ba2Y(BO3)2Cl sample. To further understand the energy transfer in this system, the decay curves of the sensitizer Ce3+ and Tb3+ in sample Ba2Y(BO3)2Cl:0.03Ce3+, 0.03Tb3+, xYb3+ (x = 0–0.3) are plotted against the concentration of Yb3+, and are shown in Fig. 5. With increasing Yb3+ concentration, the decay of Ce3+ and Tb3+(5D4) in tri-doped Ba2Y(BO3)2Cl:0.03Ce3+, 0.03Tb3+, xYb3+ sample speeds up (as shown in Fig. 5a and b), similar to those in co-doped Ba2Y(BO3)2Cl:0.03Ce3+, xYb3+ and Ba2Y(BO3)2Cl:0.03Tb3+, xYb3+. The effective decay times are calculated to be about 12.82, 9.63, 2.89, 2.60, 1.77, 1.28 ns for Ce3+ and 3.56, 3.26, 3.18, 3.06, 2.77, 2.48 ms for Tb3+, therefore the ηETE values are 36.8, 52.6, 85.8, 87.2, 91.2, 93.7% (ηETE-Ce) and 0, 8.6, 10.7, 14.0, 22.2, 30.3% (ηETE-Tb) according to the decline of Ce3+ and Tb3+ lifetime, respectively. The corresponding lifetimes and energy transfer efficiency variable curves as function of Yb3+ contents are also shown clearly in Fig. 5c and d. The ηETE-Ce values calculated on the basis of the decrease of Ce3+ lifetime do not start from 0 whereas the ηETE-Tb values do start from 0, because ηETE-Ce value is the sum of ET efficiency of Ce3+ → Tb3+ and Ce3+ → Yb3+, but ηETE-Tb is the only ET efficiency value of Tb3+ → Yb3+. Comparison with the co-doped system, it is found that the ET efficiency was higher in a tri-doped sample than those of the corresponding co-doped samples. Thus we deduced that the NIR emission intensity in Ba2Y(BO3)2Cl:0.03Ce3+, 0.03Tb3+, xYb3+ should be stronger than that of corresponding Ba2Y(BO3)2Cl:0.03Ce3+, xYb3+ or Ba2Y(BO3)2Cl:0.03Tb3+, xYb3+, which is strongly related to the energy transfer processes.
4. Conclusions
In summary, steady and dynamic photoluminescent properties and the energy transfer mechanism in the Ce3+–Tb3+–xYb3+ tri-doped Ba2Y(BO3)2Cl phosphor were systematically investigated, and four possible ET processes Ce3+ → Tb3+, Ce3+ → Yb3+, Tb3+ → Yb3+ and Ce3+ → Tb3+ → Yb3+ were proposed. As a comparison, the Ce3+/Tb3+–xYb3+ co-doped Ba2Y(BO3)2Cl were also studied. It is found that the energy transfer efficiency of Ce3+ → xYb3+ and Tb3+ → xYb3+ in the tri-doped system is larger than that of the double-doped system. The NIR emission intensity of Yb3+ in a tri-doped sample under excitation of 355 nm is 1.4 times of Ce3+–Yb3+ co-doped sample and is 23 times of Tb3+–Yb3+ co-doped phosphors with optimal composition. Moreover, Ce3+ ion significantly enlarges the absorption cross-section in the near ultraviolet region (320–390 nm) due to the allowed 4f → 5d transition and greatly enhances the NIR emission of Yb3+ ion where the c-Si solar cell exhibits the greatest spectral response. Results indicate that Ba2Y(BO3)2Cl:Ce3+, Tb3+, Yb3+ phosphor is a promising spectral convertor for enhancing photoelectric conversion efficiency of silicon solar cells.Acknowledgements
This work was supported by the high-level talent project of Northwest University, National Natural Science Foundation of China (no. 11274251), Ph.D. Programs Foundation of Ministry of Education of China (20136101110017), Technology Foundation for Selected Overseas Chinese Scholar, Ministry of Personnel of China (excellent), Natural Science Foundation of Shaanxi Province (no. 2014JM1004) and Foundation of Key Laboratory of Photoelectric Technology in Shaanxi Province (12JS094).References
- O. Morton, Nature, 2006, 443, 19–22 CrossRef CAS PubMed.
- A. Kraft, C. Wolf, J. Bartsch, M. Glatthaar and S. Glunz, Sol. Energy Mater. Sol. Cells, 2015, 136, 25–31 CrossRef CAS PubMed.
- B. M. van der Ende, L. Aarts and A. Meijerink, Phys. Chem. Chem. Phys., 2009, 11, 11081–11095 RSC.
- B. S. Richards, Sol. Energy Mater. Sol. Cells, 2006, 90, 1189–1207 CrossRef CAS PubMed.
- W. Shockley and H. J. Queisser, J. Appl. Phys., 1961, 32, 510–519 CrossRef CAS PubMed.
- M. B. Spitzer and H. P. Jenssen, Sol. Energy Mater. Sol. Cells, 2013, 108, 241–245 CrossRef CAS PubMed.
- D. Q. Chen, Y. S. Wang and M. C. Hong, Nano Energy, 2012, 1, 73–90 CrossRef CAS PubMed.
- L. Zhao, L. L. Han and Y. H. Wang, Opt. Mater. Express, 2014, 4, 1456–1464 CrossRef CAS.
- T. Trupke, M. A. Green and P. Würfel, J. Appl. Phys., 2002, 92, 1647–1668 CrossRef PubMed.
- W. J. Zhu, D. Q. Chen, L. Lei, J. Xu and Y. S. Wang, Nanoscale, 2014, 6, 10500–10504 RSC.
- Q. Y. Zhang, G. F. Yang and Z. H. Jiang, Appl. Phys. Lett., 2007, 91, 051903 CrossRef PubMed.
- V. D. Rodríguez, V. K. Tikhomirov, J. Méndez-Ramos, A. C. Yanes and V. V. Moshchalkov, Sol. Energy Mater. Sol. Cells, 2014, 122, 46–50 CrossRef PubMed.
- K. Deng, T. Gong, L. Hu, X. Wei, Y. Chen and M. Yin, Opt. Express, 2011, 19, 1749–1754 CrossRef CAS PubMed.
- H. Jing, C. F. Guo, G. G. Zhang, X. Y. Su, Z. Yang and J. H. Jeong, J. Mater. Chem., 2012, 22, 13612–13618 RSC.
- X. Y. Huang, D. C. Yu and Q. Y. Zhang, J. Appl. Phys., 2009, 106, 113521 CrossRef PubMed.
- H. Zhang, X. Y. Liu, F. Y. Zhao, L. H. Zhang, Y. F. Zhang and H. Guo, Opt. Mater., 2012, 34, 1034–1036 CrossRef CAS PubMed.
- S. Li, Z. Hou, Z. Cheng, H. Lian, P. Ma, C. Li and J. Lin, RSC Adv., 2013, 3, 5491–5497 RSC.
- N. M. Zhang, C. F. Guo, H. Jing and J. H. Jeong, Spectrochim. Acta, Part A, 2013, 116, 556–561 CrossRef CAS PubMed.
- W. J. Schipper and G. Blasse, J. Alloys Compd., 1994, 203, 267–269 CrossRef CAS.
- Q. Zhang, J. Wang, G. Zhang and Q. Su, J. Mater. Chem., 2009, 19, 7088–7092 RSC.
- Q. Y. Zhang and X. Y. Huang, Prog. Mater. Sci., 2010, 55, 353–427 CrossRef CAS PubMed.
- D. Geng, G. Li, M. Shang, C. Peng, Y. Zhang, Z. Cheng and J. Lin, Dalton Trans., 2012, 41, 3078–3086 RSC.
- Y. Li, J. Wang, W. Zhou, G. Zhang, Y. Chen and Q. Su, Appl. Phys. Express, 2013, 6, 082301 CrossRef.
- X. Liu, Y. Teng, Y. Zhuang, J. Xie, Y. Qrao, G. Dong, D. Chen and J. Qiu, Opt. Lett., 2009, 34, 3565–3567 CrossRef CAS PubMed.
- P. Vergeer, T. J. H. Vlugt, M. H. F. Kox, M. I. Den Hertog, J. P. J. M. Van der Eerden and A. Meijerink, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 71, 014119 CrossRef.
- R. Yu, S. Zhong, N. Xue, H. Li and H. Ma, Dalton Trans., 2014, 43, 10969–10976 RSC.
- J. D. Chen, H. Guo, Z. Q. Li, H. Zhang and Y. X. Zhuang, Opt. Mater., 2010, 32, 998–1001 CrossRef CAS PubMed.
- H. Lin, S. Zhou, H. Teng, Y. Li, W. Li, X. Hou and T. Jia, J. Appl. Phys., 2010, 107, 043107 CrossRef PubMed.
- J. Ueda and S. Tanabe, J. Appl. Phys., 2009, 106, 043101 CrossRef PubMed.
- E. van der Kolk, O. M. Ten Kate, J. W. Wiegman, D. Biner and K. W. Krämer, Opt. Mater., 2011, 33, 1024 CrossRef CAS PubMed.
- D. W. Cooke, R. E. Muenchausen, B. L. Bennett, K. J. McClellan and A. M. Portis, J. Lumin., 1998, 79, 185–190 CrossRef CAS.
- Y. Zhu, Z. Sun, Z. Yin, H. Song, W. Xu, Y. Wang, L. Zhang and H. Zhang, Dalton Trans., 2013, 42, 8049–8057 RSC.
- C. F. Guo, H. Jing and T. Li, RSC Adv., 2012, 2, 2119–2122 RSC.
- L. L. Han, Y. H. Wang, Y. Z. Wang, J. Zhang and Y. Tao, J. Alloys Compd., 2013, 551, 485–489 CrossRef CAS PubMed.
- I. A. A. Terra, L. J. Borrero-González, T. R. Figueredo, J. M. P. Almeida, A. C. Hernandes, L. A. O. Nunes and O. L. Malta, J. Lumin., 2012, 132, 1678–1682 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |