d-Glucosamine trimethylene dithioacetal derivatives: formation of six- and seven-membered ring amino carbasugars. Synthesis of (–)-calystegine B3†,‡
Abstract
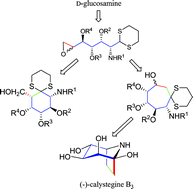
By virtue of carefully chosen protecting groups, D-glucosamine trimethylene dithioacetal derivatives were successfully oxidized to the corresponding 6-aldehydes. This methodology reverses the donor and acceptor position on a normal open chain

 Please wait while we load your content...
Please wait while we load your content...