Preparation of α-zirconium phosphate nanoplatelets with wide variations in aspect ratios
Luyi
Sun
ab,
Woong J.
Boo
a,
Hung-Jue
Sue
*a and
Abraham
Clearfield
*b
aPolymer Technology Center, Department of Mechanical Engineering, Texas A&M University, College Station, TX 77843-3123, USA. E-mail: hjsue@tamu.edu; Fax: +1 979-862-3989; Tel: +1 979-845-5024
bDepartment of Chemistry, Texas A&M University, College Station, TX 77842-3012, USA. E-mail: clearfield@mail.chem.tamu.edu; Fax: +1 979-845-2370; Tel: +1 979-845-2936
First published on 12th October 2006
Abstract
Synthetic α-zirconium phosphate (α-ZrP) layer structures have been prepared via three different approaches. By controlling the concentration of reactants, temperature, pressure, and using a complexing agent, α-ZrP with a wide variation in aspect ratios has been prepared. Synthetic α-ZrP can be easily intercalated by amines and then exfoliated in epoxy to prepare polymer nanocomposites. Nanocomposites that contain exfoliated α-ZrP nanoplatelets with wide variations in aspect ratios can be utilized as model systems to study the structure–property relationship in polymer nanocomposites.
Introduction
Since the pioneering work on nylon-6/montmorillonite (MMT) was reported in the early 1990s,1–3 inorganic layered compound/polymer nanocomposites have shown extraordinary promise as high-performance materials.4–7 Although a wide spectrum of layered materials have been studied as nanofillers for polymer matrices, MMT has been the focus of much research because of its low cost and its ability to be well-dispersed and exfoliated in a polymer matrix when using surface modification techniques.4,8 When MMT is well-exfoliated and properly incorporated into a polymer matrix, significant improvements in physical and mechanical properties can be achieved by only a small amount of MMT loading.4–7The main drawback of MMT in polymer nanocomposite applications is that it is produced via purification and modification of mined MMT. It is very difficult to achieve 100% purity, narrow particle size distribution, and controlled aspect ratio.9 Therefore, despite significant research efforts in the past, fundamental knowledge on exactly how the degree of dispersion, aspect ratio, and surface functionality influence the physical and mechanical properties of MMT-based polymer nanocomposites is still lacking. It is imperative to prepare model polymer nanocomposites that contain fully exfoliated and uniformly dispersed nanofillers with controlled aspect ratio and surface functionality to gain unambiguous fundamental understanding of mechanical behavior of polymer nanocomposites. In addition, a number of experimental and modeling studies have shown that polymer nanocomposites containing high aspect ratio plate-like nanofillers will have significant enhanced barrier properties10–12 and mechanical properties.13–17 The aspect ratio from natural clay filler is limited by the length of the clay microcrystals. To obtain higher aspect ratio nanoplatelets, new layered compounds with high aspect ratio must be prepared.
Stemming from the above concerns, α-zirconium phosphate (α-ZrP), Zr(HPO4)2·H2O, a synthetic layered compound with well-ordered structure, was prepared in this study. Crystalline α-ZrP was first discovered in 1964 by Clearfield and Stynes via refluxing amorphous α-ZrP with phosphoric acid.18 The layer structure of α-ZrP is similar to that of MMT. However, the layers are formed by zirconium atoms connected through the oxygen atoms of the phosphate groups. Each phosphate contributes three of its oxygen atoms to the formation of these layers, leaving one –OH group pointing into the interlayer space. This structure gives α-ZrP a much higher ion exchange capacity (6.64 meq g−1) than MMT. Through an acid–base reaction, where the proton is transferred from the –POH group to the nitrogen or by hydrogen bonding, these –OH groups can be readily reacted with amines.19–21 The driving force for such acid–base reactions and hydrogen bonding is much higher than the simple ion exchange reactions which occur in MMT, thus the intercalation reaction takes place much more readily in α-ZrP than in MMT. Furthermore, unlike MMT with exchangeable cations varying from layer to layer,4 which inevitably prevent a full exfoliation in MMT, the –OH groups are uniformly distributed throughout the layers of α-ZrP. Fully exfoliated epoxy/α-ZrP nanocomposites have been successfully prepared and their complete exfoliation has been verified by both TEM imaging and interlayer distance modeling work.9,22
Since α-ZrP can be prepared through a synthetic route, the manipulation on the lateral (i.e., the length) dimension of the α-ZrP to achieve high aspect ratios becomes rather straightforward. As a result, it becomes possible to fundamentally investigate how the aspect ratio of the nanoplatelets would affect the physical and mechanical behavior of polymer nanocomposites. Control of the lateral layer dimensions can be used to prepare different grades of polymer nanocomposites to match different commercial requirements. Finally, it may allow the polymer nanocomposite to exhibit the highest possible mechanical strength and barrier properties.
Because of its ease of exfoliation and aspect ratio control, crystalline α-ZrP was selected as a model nanofiller to prepare polymer nanocomposites. In the present study, three different approaches were carried out to prepare α-ZrP with wide variations in aspect ratio.
Experimental
Materials
Zirconyl chloride (ZrOCl2·8H2O, 98%, Aldrich), phosphoric acid (85%, EM Science), hydrofluoric acid (48%, EMD Chemicals), and tetra-n-butylammonium hydroxide (TBA, Aldrich) were used as received. A series of commercial polyoxyalkyleneamines, Jeffamine M300, M500, M600, and M715 with a reported average molecular weight of 300, 500, 600, and 715, respectively (Huntsman Chemical) were used to intercalate α-ZrP.Preparation of α-ZrP
Three different approaches were designed to prepare α-ZrP with wide variations in aspect ratio. In each of the three approaches, the reaction time and/or concentrations of reactants were also varied to obtain α-ZrP with the designed range of aspect ratios.Characterizations
X-Ray powder diffraction (XRD) patterns were recorded using a Bruker D8 diffractometer with Bragg–Brentano θ–2θ geometry (40 kV and 40 mA), using a graphite monochromator with Cu-Kα radiation.Scanning electron microscopy (SEM) images were acquired on a Zeiss Leo 1530 VP Field Emission-SEM (FE-SEM). The samples were sputter coated with a thin layer (ca. 3 nm) of Pt/Pd (80/20) prior to the SEM imaging.
Results and discussion
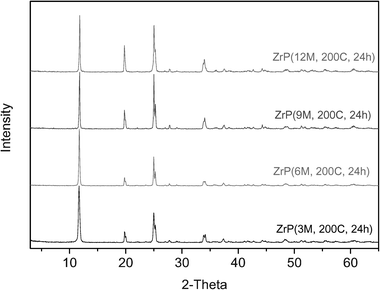
Crystalline α-ZrP was first prepared via the refluxing method.18 It is anticipated that the crystallinity will increase with the increasing concentration of phosphoric acid, and the aspect ratio of the products will increase correspondingly. This is validated by the XRD patterns and SEM images of ZrP(3M), ZrP(6M), ZrP(9M), and ZrP(12M) shown in Fig. 1 and 2, respectively. The length dimension of the α-ZrP nanoplatelet increases from ∼60 nm to ∼200 nm. However, it becomes difficult to obtain larger α-ZrP platelets through this approach. | ||
| Fig. 1 XRD patterns of α-ZrP based on the refluxing method. Values in parenthesis are the concentration of H3PO4 used in the preparation. | ||
 | ||
| Fig. 2 SEM images of (a) ZrP(3M), (b) ZrP(6M), (c) ZrP(9M), and (d) ZrP(12M). | ||
In the refluxing method, the concentration of phosphoric acid up to 12.0 M and a longer refluxing time does not effectively increase the aspect ratio of α-ZrP.23 Meanwhile, it is not practical to further increase refluxing temperature. Therefore, a hydrothermal method was adopted to further increase the reaction temperature, as well as to assess the effect of pressure on the crystal growth process. Selected XRD patterns and SEM images of samples from Approach II are presented in Fig. 3 and 4, respectively. Compared with α-ZrP samples prepared from Approach I, the samples from Approach II show a much enhanced crystallinity, especially for the samples obtained from low concentrations of phosphoric acid. With the increase of reaction time, their crystallinity was further increased, but not significantly. The aspect ratio of the samples after 5 hours of reaction is just slightly higher than the corresponding samples from Approach I. The largest nanoplatelet after the 5 hours of reaction is ∼300 nm. However, after a longer reaction time of 24 hours, the crystals grew to a much higher aspect ratio. As shown in Fig. 4a, the length of ZrP(3M-200C-24h) is ∼400 nm, and the length of ZrP(12M-200C-24h) reaches ∼1 μm. To be noted, the α-ZrP prepared via the hydrothermal method has a narrow size distribution compared with the ones prepared from the refluxing method. Such a narrow size distribution may benefit the fundamental studies on the structure–property relationship of polymer nanocomposites.
 | ||
| Fig. 3 XRD patterns of crystalline α-ZrP based on the hydrothermal method (24 hours). Values in parenthesis give the H3PO4 concentration, temperature and time. | ||
 | ||
| Fig. 4 SEM images of (a) ZrP(3M-200C-24h), (b) ZrP(6M-200C-24h), (c) ZrP(9M-200C-24h), and (d) ZrP(12M-200C-24h). | ||
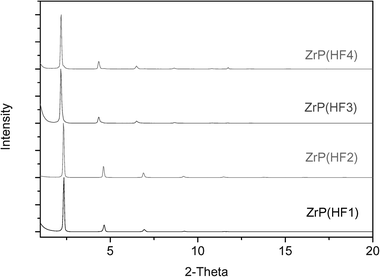
Through the above two approaches, the α-ZrP nanoplatelets with aspect ratios from ∼100 to over 1200 have been prepared by controlling the concentrations of reactants and duration of reactions. To further increase the aspect ratio of the α-ZrP nanoplatelets, an HF method, which was developed by Alberti and Torracca,24 was adopted. HF is a good complexing agent for zirconium. Therefore, when the concentration of HF is high enough, precipitation by phosphoric acid is inhibited. With the evaporation of HF, α-ZrP starts to precipitate gradually and such a slow precipitation helps to grow large crystals.24Fig. 5 and 6 show the XRD patterns and SEM images of ZrP(HF1), ZrP(HF2), ZrP(HF3), and ZrP(HF4), respectively. Compared with the XRD patterns of α-ZrP from Approaches I and II, it is clear that the α-ZrP obtained from the HF method has a much higher crystallinity. Correspondingly, the aspect ratio of the samples increase to over 2000 for ZrP(HF1) and over 4000 for ZrP(HF4). Compared with the samples from the hydrothermal method, the α-ZrP crystals prepared from HF method have the highest aspect ratio. However, their size distribution is also wide.
 | ||
| Fig. 5 XRD patterns of α-ZrP based on the HF method. Values in parenthesis give the ratio of HF to Zr. | ||
 | ||
| Fig. 6 SEM images of (a) ZrP(HF1), (b) ZrP(HF2), (c) ZrP(HF3), and (d) ZrP(HF4). | ||
From the above three approaches, α-ZrP platelets with an aspect ratio from 100 to over 4000 have been successfully prepared. The length and the reaction yields of the α-ZrP prepared are summarized in Table 1. By exfoliating such model layered compounds to prepare polymer nanocomposites, the fundamental structure–property relationship of polymer nanocomposites, such as how the aspect ratio of nanofillers can affect the physical and mechanical properties, can be clearly addressed. It is noted that much larger α-ZrP crystals can be prepared by slowing down the HF evaporation rate,25 which can be used to prepare polymer nanocomposites containing much higher aspect ratio nanofillers. However, some modeling work13 has suggested that ultrahigh aspect ratios cannot bring about a dramatic jump in modulus due to the significantly reduced volume fraction of ultrahigh aspect ratio nanoplatelets that can be incorporated in a polymer matrix. Another negative impact from the use of ultrahigh aspect ratio nanoplatelets is the undesirable huge increase in viscosity.16 When ZrP(3M) and ZrP(HF1) are mixed with the epoxy monomer at 1.0 vol%, respectively, the viscosity of the epoxy/ZrP(HF1) mixture is much higher than that of the ZrP(3M) case.26
| Sample | Yield (%) | Typical particle length/nm |
|---|---|---|
| ZrP(3M) | 96.4 | 50–100 |
| ZrP(6M) | 87.3 | 100–200 |
| ZrP(9M) | 85.8 | 100–200 |
| ZrP(12M) | 86.6 | 150–300 |
| ZrP(HT3M-200-5) | 85.6 | 100–200 |
| ZrP(HT6M-200-5) | 90.7 | 150–250 |
| ZrP(HT9M-200-5) | 98.0 | 150–250 |
| ZrP(HT12M-200-5) | 97.3 | 200–400 |
| ZrP(HT3M-200-24) | 89.3 | 300–500 |
| ZrP(HT6M-200-24) | 97.1 | 600–800 |
| ZrP(HT9M-200-24) | 96.0 | 800–1000 |
| ZrP(HT12M-200-24) | 93.7 | 1000–1200 |
| ZrP(HF1) | 83.5 | 1000–2000 |
| ZrP(HF2) | 72.0 | 1000–3000 |
| ZrP(HF3) | 53.5 | 1500–3500 |
| ZrP(HF4) | 41.8 | 2000–4000 |
The α-ZrP can be easily intercalated by amines, such as Jeffamines.9 It has been found that it is easier to intercalate low crystallinity α-ZrP.27 When intercalating α-ZrP with low crystallinity, a long chain amine, such as M715, can effectively increase the interlayer space up to ∼76 Å.9,27 In contrast, to intercalate α-ZrP that possesses high crystallinity, it is easer to achieve a full intercalation with a relatively short chain amine, such as M300. Two examples, ZrP(3M) interlaced by M300, M500, M600, and M715, and four α-ZrP samples from the HF method intercalated by M300 are shown in Fig. 7 and 8, respectively. In addition, ZrP can also be directly exfoliated by TBA.28
 | ||
| Fig. 7 XRD patterns of ZrP(3M) intercalated by Jeffamines M300, M500, M600, and M715 at a 1 : 2.0 molar ratio. | ||
 | ||
| Fig. 8 XRD patterns of ZrP(HF1), ZrP(HF2), ZrP(HF3), and ZrP(HF4) intercalated by M300 at a 1 : 2.0 molar ratio. | ||
Several approaches have been developed to prepare exfoliated epoxy/α-ZrP nanocomposites based on the effective intercalation and exfoliation processes described above. Our recent efforts have indicated that Jeffamine9 or TBA,29 or a mixture of both,26 are effective to achieve full exfoliation of α-ZrP nanoplatelets in epoxy. Systematic studies on the structure–property relationship based on the fully exfoliated epoxy nanocomposites will be reported in the near future.
Conclusions
α-ZrP with wide variations in aspect ratios were prepared via three different approaches. They can be easily intercalated by amines, and then exfoliated in epoxy to prepare polymer nanocomposites. Such polymer nanocomposites can be used as a model system to study the structure–property relationship of polymer nanocomposites.Acknowledgements
This work was sponsored by Specialty Minerals Inc. and National Science Foundation DMR-0332453 for which grateful acknowledgement is made. The SEM acquisition was supported by the National Science Foundation under Grant No. DBI-0116835. L. Y. Sun thanks Dr Deyuan Kong for valuable discussions.References
- A. Usuki, Y. Kojima, M. Kawasumi, A. Okada, Y. Fukushima, T. Kurauchi and O. Kamigaito, J. Mater. Res., 1993, 8, 1179–1184 CrossRef CAS.
- Y. Kojima, A. Usuki, M. Kawasumi, A. Okada, Y. Fukushima, T. Kurauchi and O. Kamigaito, J. Mater. Res., 1993, 8, 1185–1189 CrossRef CAS.
- Y. Kojima, A. Usuki, M. Kawasumi, A. Okada, T. Kurauchi and O. Kamigaito, J. Polym. Sci., Part A: Polym. Chem., 1993, 31, 983–986 CrossRef CAS.
- M. Alexandre and P. Dubois, Mater. Sci. Eng., R, 2000, 28, 1–63 Search PubMed.
- E. P. Giannelis, Adv. Mater., 1996, 8, 29–35 CAS.
- S. S. Ray and M. Okamoto, Prog. Polym. Sci., 2003, 28, 1539–1641 CrossRef CAS.
- S. J. Ahmadi, Y. D. Huang and W. Li, J. Mater. Sci., 2004, 39, 1919–1925 CrossRef CAS.
- L. F. Drummy, H. Koerner, K. Farmer, A. Tan, B. L. Farmer and R. A. Vaia, J. Phys. Chem. B, 2005, 109, 17868–17878 CrossRef CAS.
- H.-J. Sue, K. T. Gam, N. Bestaoui, N. Spurr and A. Clearfield, Chem. Mater., 2004, 16, 242–249 CrossRef CAS.
- C. Lu and Y.-W. Mai, Phys. Rev. Lett., 2005, 95, 088303 CrossRef.
- M. A. Osman, V. Mittal and H. R. Lusti, Macromol. Rapid Commun., 2004, 25, 1145–1149 CrossRef CAS.
- M. A. Osman, V. Mittal, M. Morbidelli and U. W. Suter, Macromolecules, 2004, 37, 7250–7257 CrossRef CAS.
- N. Sheng, M. C. Boyce, D. M. Parks, G. C. Rutledge, J. I. Abes and R. E. Cohen, Polymer, 2004, 45, 487–506 CrossRef CAS.
- J.-J. Luo and I. M. Daniel, Compos. Sci. Technol., 2003, 63, 1607–1616 CrossRef CAS.
- J. I. Weon and H.-J. Sue, Polymer, 2005, 46, 6325–6334 CrossRef CAS.
- D. P. N. Vlasveld, M. de Jong, H. E. N. Bersee, A. D. Gotsis and S. J. Picken, Polymer, 2005, 46, 10279–10289 CrossRef CAS.
- M. Okoshi and H. Nishizawa, Fire Mater., 2004, 28, 423–429 CrossRef CAS.
- A. Clearfield and J. A. Stynes, J. Inorg. Nucl. Chem., 1964, 26, 117–129 CrossRef CAS.
- A. Clearfield, Annu. Rev. Mater. Sci., 1984, 14, 205–229 CrossRef CAS.
- A. Clearfield, in Progress in Intercalation Research, ed. W. Müller-Warmuth and R. Schollhorn, Kluwer, Dordrecht, 1994, pp. 240–263 Search PubMed.
- A. Clearfield and U. Costantino, in Comprehensive Supramolecular Chemistry, ed. G. Alberti and T. Bein, Elsevier, Oxford, UK, 1996, vol. 7, pp. 107–149 Search PubMed.
- H.-J. Sue, K. T. Gam, N. Bestaoui, A. Clearfield, M. Miyamoto and N. Miyatake, Acta Mater., 2004, 52, 2239–2250 CrossRef CAS.
- A. Clearfield, L. Kullberg and A. Oskarsson, J. Phys. Chem., 1974, 78, 1150–1153 CrossRef CAS.
- G. Alberti and E. Torracca, J. Inorg. Nucl. Chem., 1968, 30, 317–318 CrossRef CAS.
- G. Alberti, U. Costantino, S. Allulli and M. A. Massucci, J. Inorg. Nucl. Chem., 1975, 37, 1779–1786 CrossRef.
- L. Sun, W. J. Boo, C. Tien, A. Clearfield and H.-J. Sue, Adv. Mater. Search PubMed , submitted.
- L. Sun, W. J. Boo, R. L. Browning, H.-J. Sue and A. Clearfield, Chem. Mater., 2005, 17, 5606–5609 CrossRef CAS.
- D. M. Kaschak, S. A. Johnson, D. E. Hooks, H.-N. Kim, M. D. Ward and T. E. Mallouk, J. Am. Chem. Soc., 1998, 120, 10887–10894 CrossRef CAS.
- H. J. Boo, L. Sun, J. Liu, A. Clearfield, H.-J. Sue, M. J. Mullins and H. Pham, Compos. Sci. Technol., 2006 DOI:10.1016/j.compscitech.2006.08.012.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2007 |
