Hot off the press
Hot off the Press highlights recently published work for the benefit of our readers. Our contributors this month have focused on a semi-synthetic approach to identify kinase substrates. New contributors are always welcome. If you are interested please contact molbiosyst@rsc.org for more information, we’d like to hear from you.
A semi-synthetic approach to identify kinase substrates
Reviewed by: Di Cai, UT Southwestern Medical Center, USA.
Protein phosphorylation is very important for different signal transductions in cells. However, it is very challenging to biochemically identify the substrates of kinase of interest (KOI), due to the fact that most phosphoproteins are of low abundance, substoichiometrically phosphorylated and the low affinity of kinase-substrate interaction.
Allen and co-workers at UCSF have previously (JACS 2005, 127, 5288–5289) shown a bio-orthogonal affinity purification of direct kinase substrates. The KOI was engineered to accept the ATP analog A*TPγS, which is an N6-modified ATPγS and can not be recognized by wild-type kinases. The engineered KOI catalyzed thiophosphorylation of its direct substrates, which was then alkylated of p-nitrobenzylmesylate (PNBM), creating an epitope for specific antibody recognition.
In the latest Nature Methods paper, the same group reported three advances in developing this semi-synthetic reaction scheme: (1) a monoclonal antibody was generated to specifically recognize the PNBM alkylated thiophosphate group irrespective of the peptide sequence; (2) analysis of each reaction step for specificity and applicability to 13 kinase substrate pairs; (3) capability to label substrate in cells expressing endogenous level of analog sensitive Erk2, allowing purification and identification of a direct Erk2 substrate.
Finally, this semi-synthetic epitope tagging may serve as a general strategy to label and affinity purify the substrates of different kinases, providing a new route to study phosphoproteomics.
Jasmina J. Allen, Manqing Li, Craig S. Brinkworth, Jennifer L. Paulson, Dan Wang, Anette Habner, Wen-Hai Chou, Roger J. Davis, Alma L. Burlingame, Robert O. Messing, Carol D. Katayama, Stephen M. Hedrick, Kevan M. Shokat, Nature Methods, 2007, 4(6), 511–6.
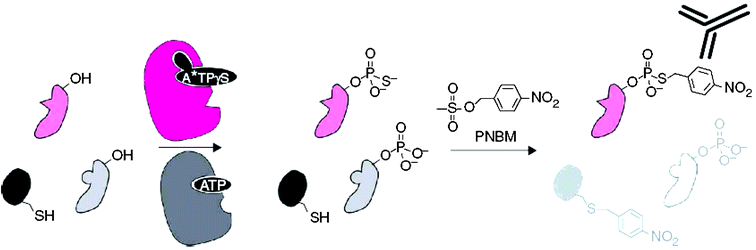 | ||
| Fig. 1 Strategy for labeling individual kinase substrates. First, an analog sensitive (as) kinase (magenta) uses N6-alkylated ATPγS(A*TPγS) to thiophosphorylate its substrates (pale magenta). In a second step, alkylation with PNBM yields thiophosphate esters and thioethers. Only thiophosphate is recognized by the specific antibody α-hapten-IgG. Allen, et al., Nature Methods, 2007. | ||
Hot off the RSC press
Snapshots of specialising cells
Reviewed by: Rachel Warfield, Royal Society of Chemistry, Cambridge, UK
Researchers in Germany have used infrared spectroscopy to spot stem cells as they change into new cell types.
Christoph Krafft, at the Dresden University of Technology, and colleagues wanted to develop a spectroscopic technique that would tell if stem cells were differentiating, a process in which they become a specialised type of cell, such as a skin cell or a white blood cell.
Stem cells are used in medicine to restore damaged tissues and are being investigated to find new treatments. Krafft explained: 'A frequent problem in stem cell research is to determine the type and differentiation state of the cells. The standard techniques depend on antibodies, which recognise cell-specific antigens. However, antibodies are not available for each cell type or differentiation state and the antigen-antibody binding is sometimes weak or not specific enough.'
Krafft and his team coupled an infrared (IR) spectrometer with a microscope so they could locate stem cells and measure their IR spectra. They grew some of the cells in a medium designed to stimulate them to turn into bone-forming cells, called osteoblasts. The IR spectra from these cells were different from those of non-stimulated cells. Both sets of spectra were used to train a computer model to distinguish between the cell groups.
The team used the IR microscope and computer model to produce images of stem cells; stimulated and non-stimulated cells appeared as different colours, showing whether they had differentiated (Fig. 2). The technique also showed stimulated cells that had started to produce calcium phosphate salts for bone formation. Krafft explained that he hopes to develop the research to find out more about how stem cells form new bone.
Christoph Krafft, Reiner Salzer, Sebastian Seitz, Christina Ern and Matthias Schieker, Analyst, 2007, 132, 647
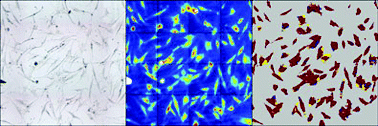 | ||
| Fig. 2 In a computer image non-stimulated stem cells (left) appear red when their IR spectra (centre) are analysed (right); stimulated cells appear blue. | ||
Delivering RNA with pinpoint precision
Reviewed by: Danièle Gibney, Royal Society of Chemistry, Cambridge.
A microchip that allows controlled delivery of genetic material to cell cultures will be a major advance for the life sciences, say US bioengineers.
The chip, a microelectrode array developed by Tilak Jain and Jit Muthuswamy of the Arizona State University in Tempe, delivers electronic pulses to specific areas of a cell culture grown on top. The cell membranes in those areas become porous and the cells absorb any genetic material present in the surrounding medium.
Delivering genetic material to cells, known as transfection, is an important process in understanding gene function and drug discovery. The transfected material is designed to either overexpress or silence genes in target cells and the effect of the change in gene activity on the cells is then studied.
Muthuswamy explained that a big advantage of his array is that multiple types of genetic material can be transfected sequentially and in different areas of the cell culture. In this way, combinations of overexpressed or silenced genes can be studied. Doing this in one culture is important: ‘inter-culture variabilities are eliminated,' said Muthuswamy. ‘Also, you can assess interactions between transfected cells and control cells.’
‘This method is easier to use and quicker than previous methods,' Muthuswamy said. But, a drawback is that different RNAs or DNAs to be transfected would have to be sequentially applied to the cells and washed off again following transfection—a time-consuming process. To make the array more suitable for high-throughput studies, Muthuswamy plans to include a microfluidic system to deliver the genetic material to the cell culture.
Muthuswamy intends to use his technology in his own research area of brain injury. 'We can test the role of specific genes in repair of damaged neurons,' he said. He is currently taking the first step, applying his method to transfect neuron cells.
Tilak Jain and Jit Muthuswamy, Lab Chip, 2007, DOI: 10.1039/b707479d
 | ||
| Fig. 3 Cells exposed to higher voltage pulses from the microchip (right) show higher uptakes of genetic material and show up green (left) | ||
| This journal is © The Royal Society of Chemistry 2007 |
