Permanent magnetism in apoferritin-encapsulated Pd nanoparticles†
Miguel
Clemente-León
a,
Eugenio
Coronado
*a,
Alejandra
Soriano-Portillo
a,
Natividad
Gálvez
*b and
José M.
Domínguez-Vera
b
aICMol Instituto de Ciencia Molecular, Universidad de Valencia, Polígono de la Coma, s/n, 46980, Paterna, Spain. E-mail: eugenio.coronado@uv.es; Fax: (+34)963543273; Tel: (+34)963544415
bDpto. Química Inorgánica, Facultad de Ciencias, Universidad de Granada, 18071, Granada, Spain. E-mail: ngalvez@ugr.es; Fax: (+34)958248526
First published on 3rd November 2006
Abstract
Pd nanoparticles have been prepared within the apoferritin cavity. X-Ray powder diffraction, transmission electronic microscopy and magnetization measurements have been used for characterizing the nanoparticles. The nanoparticles exhibit permanent magnetism at room temperature.
The onset of ferromagnetism in normally non-magnetic materials like Pd and late 4d transition metals is attracting great attention from both the theoretical and experimental points of view. Pd is a very special case as it lies close to a ferromagnetic instability. Thus, although a free atom has a [Kr] 4 d10 configuration and is non-magnetic, in bulk (fcc structure) its electronic structure is such that the density of states, N(E), just below the Fermi level, EF, shows a sharp peak with N(EF) = 1.23 states eV−1 spin−1 atom−1. As a result this metal is close to fulfilling the Stoner criterion for ferromagnetism,1N(EF)I > 1 (I stands for the exchange integral, typically 0.71 eV for Pd). Still, bulk Pd only shows enhanced Pauli paramagnetism, but not ferromagnetism. In order to observe ferromagnetism N(EF) has to be increased.
One possible approach to increase N(EF) is that of preparing Pd nanostructures (ultrathin films or nanoparticles) since in these low dimensional systems confinement, non-cubic local field symmetry and surface-induced anisotropy effects are enhanced.2 In 1997, Taniyama et al. found experimental evidence for a magnetic moment in gas-evaporated Pd nanoparticles with average radius below 7 nm.3 A more complete study was carried out by these authors in 2003 in which surface ferromagnetism was experimentally observed and attributed to the (100) facets of the particle.4 The Curie temperature of these nanoparticles was higher than 400 K. More recently Hernando et al. found hysteresis loops at room temperature for alkylammonium protected5 and thiol capped6 Pd nanoparticles in the size range between 1.2 and 2.4 nm.7 The origin of this magnetic behaviour was explained on the basis of two different mechanisms: (i) when the nanoparticle is a pure metallic Pd cluster without covalent Pd–S bonds at the surface or without an oxide passivation layer, ferromagnetism occurs which can be attributed to the increase in the density of states near the Fermi level; (ii) when a depletion shell structure is formed (with Pd–S or Pd–O chemical bonds at the surface of the nanoparticle), an increase in the 4d density of holes induced by bonding and a localization of the magnetic moment at these Pd sites occurs, leading to highly anisotropic permanent magnetism in the sample.7
As these magnetic phenomena are quite new and controversial and very few examples are known, it will be of interest to study the magnetic properties of Pd nanoparticles synthesized by other methods. One possible molecular approach to obtaining well-insulated metallic nanoparticles is that of using the apoferritin molecule as a nanoreactor.8 Apoferritin consists of a spherical protein shell composed of 24 subunits surrounding an aqueous cavity with a diameter of about 8 nm.9 The high stoichiometry binding of some metal ions to the inner cavity wall of apoferritin8 and the capacity of these bonded metal ions to be reduced by the appropriate chemical reagent gives rise to the nucleation of zero-valent metallic nanoparticles. This method has been used very recently for the preparation of Pd,10 Cu11 and superparamagnetic Ni and Co nanoparticles.12 In the case of the apoferritin-encapsulated Pd nanoparticles no magnetic properties were measured. In this communication we report a complete structural and magnetic characterization of apoferritin encapsulated Pd nanoparticles.
Apoferritin (Sigma-Aldrich, 5 mg ml−1) was incubated with 500 equivalents of K2PdCl4 for 1 h at room temperature and then NaBH4 (20 equivalents) was added. The resulting black solution was exhaustively dialyzed against water at 4 °C, chromatographed (Sephadex G-25), and the apoferritin-containing fractions were isolated. Two representative TEM images are shown in Fig. 1.13 Discrete electron-dense cores which are generally spherical in shape are clearly observed. The mean diameter was statistically measured to be 2.4 ± 1 nm by sampling 100 particles. Energy dispersive spectroscopy (EDS) confirmed that the particles contained Pd (Fig. 1c). Pd was not detected outside the particles. TEM images of samples negatively stained with uranyl acetate (to visualize the protein shell) confirmed that the particles were actually produced within the apoferritin interior (Fig. 1a, inset). The presence of the apoferritin coat prevents irreversible aggregation of the metal particles and their subsequent precipitation.
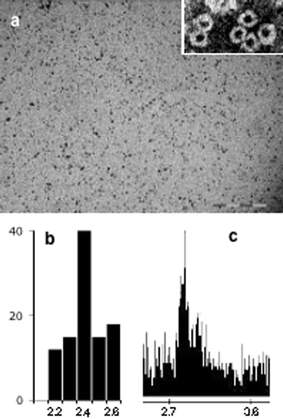 | ||
| Fig. 1 (a) TEM image of Pd nanoparticles. Scale bar is 20 nm. Inset: negatively stained (uranyl acetate) TEM image, showing the electron-transparent protein shell. (b) Size histogram. (c) EDS spectrum. | ||
The XRD pattern of the Pd nanoparticle powder (Fig. 2) shows three broad peaks centered around 2θ = 40, 46 (shoulder) and 68°,13 corresponding to the fcc Pd (100), (200) and (220) reflections, respectively.14 Likewise, a set of broad peaks (2θ = 10° and 20°) attributable to apoferritin is also noticeable. No peak that could be associated with PdO was observed. A particle mean diameter of 2.4 ± 0.3 nm was calculated from the 100, 200 and 220 reflections using the Scherrer formula. This value is in good agreement with that measured by TEM.
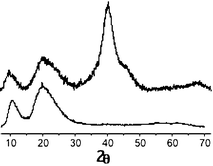 | ||
| Fig. 2 Powder XRD of the Pd nanoparticles (upper) and apoferritin (lower). | ||
The magnetic properties of powdered samples of the Pd–apoferritin nanoparticles are reported in Fig. 3 and 4.13 Marked hysteresis loops with coercivity are observed from 2 to 300 K, indicating permanent magnetism even at room temperature. The magnetization vs.H curves above 100 K show a sharp increase at low fields and a tendency to saturation at high fields, which is more pronounced at the higher temperatures (200 and 300 K). In this range these curves do not appreciably change with temperature (Fig. 3). At low temperatures (below 100 K) the magnetization is far from saturation, and the curves show a significant temperature dependence (Fig. 4). Thus, in the 2–100 K range the maximum values of magnetization decrease by one order of magnitude when the temperature increases. These results are indicative of the coexistence of blocked ferromagnetic entities, which dominate the high temperature region, with superparamagnetic particles and paramagnetic atoms, which dominate the low temperature behaviour.
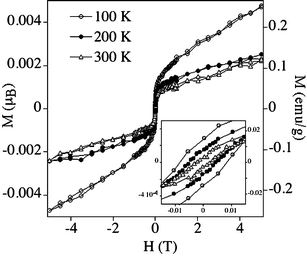 | ||
| Fig. 3 Hysteresis loop of magnetisation of the Pd nanoparticles at 100, 200 and 300 K. | ||
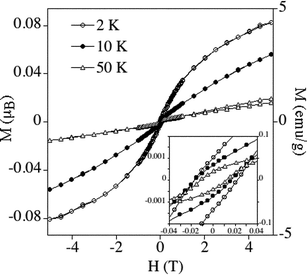 | ||
| Fig. 4 Hysteresis loop of magnetisation of the Pd nanoparticles at 2, 10 and 50 K. | ||
The temperature dependence of the coercive fields shows a decrease of the coercive field (Hc) with T from 197 G at 2 K to 26 G at 300 K. At 350 K the coercivity vanishes. Thus, 350 K should rather correspond to a blocking temperature, Tb. A linear dependence of Hc on T1/2 is found. This agrees with the expected behaviour below Tb for superparamagnetic nanoparticles. In this case the temperature dependence of coercivity obeys the equation Hc = Hci[1 − (T/Tb)1/2], where Hc is the coercive field and Hci the zero temperature coercivity.15 From the fitting to this equation a Tb value of 353 K is obtained which is in very good agreement with the experimental value. By using the relationship Tb = k〈V〉/30kB, where kB is the Boltzmann constant and 〈V〉 the particle average volume (8 × 10−27 m3 for a diameter of 2.5 nm), the effective anisotropy k of the Pd particles is estimated to be k = 1.5 × 107 J m−3, which is of the same order of magnitude as that obtained for surfactant protected Pd nanoparticles.5
Another parameter of interest is the permanent magnetic moment of the nanoparticles. This value can be roughly estimated assuming that it is uniformly distributed over all the particles and over all the Pd atoms of each particle. A value close to 10−3 Bohr magnetons per Pd is obtained from saturation of magnetization at 300 K. Such a low value seems to indicate that only a small fraction of atoms exhibit a permanent magnetic moment and ferromagnetism.
The DC magnetic susceptibility (χ) of the nanoparticles shows a clear dependence on the temperature, exhibiting a continuous increase upon cooling. This behaviour is different from that observed for Pd bulk metal and for the alkylammonium-protected 2 nm nanoparticles formed by pure metallic Pd clusters, which exhibit almost constant χ values (see ESI†). This constitutes additional evidence for the presence of superparamagnetic and paramagnetic contributions to the magnetism of our sample.
All the above results are clear evidence of permanent magnetism in the Pd–apoferritin nanoparticles. Since ferromagnetism in Pd nanoparticles is a new and rare phenomenon not yet well understood, it will be of interest to compare our results with those recently reported by Taniyama et al.4 and by Hernando et al.5–7 The main difference between our results and the previous reports deals with the temperature dependence of the hysteresis loops. Thus, in contrast to what has been observed in the Pd–apoferritin nanoparticles, the hysteresis loops of most of the Pd nanoparticles already reported do not appreciably change with temperature. An exception to this rule has been observed in the 2 nm Pd nanoparticles surrounded by an oxide passivation layer at the surface. In this particular case the M(H) values increase by one order of magnitude upon cooling from 300 to 5 K.7 This variation is similar to that of the Pd–apoferritin nanoparticles. Another similarity concerns the value of the permanent magnetic moment which in both cases is close to 10−3 Bohr magnetons per Pd at room temperature, increasing to 10−2 at low temperatures.
These observations suggest that the Pd nanoparticles encapsulated by the apoferritin are surrounded by a small Pd oxide shell around the metallic Pd core. Thus, although XRD data indicate that the nanoparticles are formed mostly by fcc Pd, the presence of the passivation shell cannot be excluded since there are no protecting groups around the nanoparticles. In fact, the size of the Pd nanoparticles (2.4 nm) is much smaller than that of the apoferritin cavity (8 nm). Moreover, the contrasted TEM images showed a centered metal core with respect to the apoferritin shell. Therefore, most of the Pd atoms should be inside the cavity in contact with solvent water molecules rather than the apoferritin wall. A deeper study with other techniques (XPS, XANES and EXAFS) is in progress in order to understand the degree of oxidation and coordination of the Pd surface atoms.
According to the size and structural features of these nanoparticles, the mechanism proposed to account for the permanent magnetism in the Pd nanoparticles covered by an oxide layer should be still valid:7 at low temperatures the moments are fixed thanks to an increase in the 4d density of holes localized by O bonded atoms, whereas at high temperatures the effects increasing the density of states near the Fermi level become more important and account for the ferromagnetism observed at room temperature.
In conclusion, we have shown that Pd nanoparticles containing ca. 500 atoms (2.4 nm size) encapsulated within the apoferritin cavity show permanent magnetism up to room temperature. The magnetic behaviour of these nanoparticles suggests a coexistence of blocked ferromagnetic entities with superparamagnetic particles and paramagnetic atoms. The Pd–apoferritin system constitutes one of the few examples reported in the literature presenting such interesting and recently discovered behaviour for Pd nanoparticles. The flexibility of this molecular method to prepare well-insulated Pd nanoparticles of variable size and composition could afford the opportunity to study the variables that affect this not-completely-understood phenomenon. In fact, preliminary results indicate that Pd nanoparticles containing 250 and 1000 atoms inside the apoferritin cavity exhibit paramagnetic behaviour, while smaller Pd nanoparticles with 100 atoms exhibit hysteresis loops up to 100 K.
Acknowledgements
Financial support from the EU (MAGMANet NoE), the Spanish MEC (Projects MAT2004-3849 and CTQ2005-07906) and the Generalitat Valenciana are gratefully acknowledged.Notes and references
- H. Chen, N. E. Bremer and J. Callaway, Phys. Rev. B, 1989, 40, 1443 CrossRef CAS and references therein.
- (a) M. J. Zhu, D. M. Bylander and L. Kleinman, Phys. Rev. B, 1990, 42, 2874 CrossRef CAS; (b) O. Eriksson, R. C. Alberts and A. M. Boring, Phys. Rev. Lett., 1991, 66, 1350 CrossRef CAS; (c) S. Blügel, Europhys. Lett., 1992, 18, 257 CrossRef.
- T. Taniyama, E. Ohta and T. Sato, Europhys. Lett., 1997, 38, 195 CrossRef CAS.
- T. Shinohara, T. Sato and T. Taniyama, Phys. Rev. Lett., 2003, 91, 197201 CrossRef CAS.
- B. Sampedro, P. Crespo, A. Hernando, R. Litrán, J. C. Sánchez-López, C. López-Cartes, A. Fernández, J. Ramírez, J. González-Calbet and M. Vallet, Phys. Rev. Lett., 2003, 91, 23203.
- A. Hernando, B. Sampedro, R. Litrán, T. C. Rojas, J. C. Sánchez-López and A. Fernández, Nanotechnology, 2006, 16, 1449 CrossRef.
- R. Litrán, B. Sampedro, T. C. Rojas, M. Multigner, J. C. Sánchez-López, P. Crespo, C. López-Cartes, M. A. García, A. Hernando and A. Fernández, Phys. Rev. B, 2006, 73, 054404 CrossRef.
- S. Pead, E. Durrant, B. Webb, C. Larsen, D. Heaton, J. Johnson and G. D. Watt, J. Inorg. Biochem., 1995, 59, 15 CrossRef CAS.
- (a) P. M. Harrison and P. Arosio, Biochim. Biophys. Acta, 1996, 1275, 161 CrossRef CAS; (b) P. M. Proulx-Curry and N. D. Chaspeen, Coord. Chem. Rev., 1995, 144, 347 CrossRef CAS.
- T. Ueno, M. Suzuki, T. Goto, T. Matsumoto, K. Nagayama and Y. Watanabe, Angew. Chem., Int. Ed., 2004, 43, 2527 CrossRef CAS.
- N. Galvez, P. Sanchez and J. M. Domínguez-Vera, Dalton Trans., 2005, 2492 RSC.
- N. Gálvez, P. Sánchez, J. M. Domínguez-Vera, A. Soriano-Portillo, M. Clemente-León and E. Coronado, J. Mater. Chem., 2006, 16, 2757 RSC.
- The samples used for transmission electronic microscopy (TEM) study were prepared by diluting the dialyzed solutions of Pd–apoferritin with milli-Q water and then placing a drop onto a carbon-coated Cu grid and drying it in a glove box. The average particle sizes and the standard deviations were estimated from TEM image analysis of 100 particles. Electron micrographs were taken with a Philips CM-20 HR analytical electron microscope operating at 200 keV. The nanoparticles were characterized by powder X-ray diffraction (XRD) with a Bruker D8 ADVANCE diffractometer using CuKα radiation. Magnetic susceptibility measurements were performed on evaporated samples using a magnetometer (Quantum Design MPMS-XL-5) equipped with a SQUID sensor. The diamagnetism from the holder was corrected by measuring the empty holder at the same conditions (magnetic field and temperature) used for the measurement of the Pd nanoparticles. Diamagnetism from apoferritin was also corrected. The molecular weight used for magnetic calculations was that obtained from the sum of the apoferritin molecular weight (475000 g mol−1) plus the atomic weight of 500 Pd.
- X-Ray Powder Diffraction File JCPDS-ICDD (Joint Committee on Powder Diffraction Standards-International Centre for Diffraction Data, Swarthmore, PA) 46-1043 for Pd fcc and 46-1211 for PdO..
- M. E. McHenry, S. A. Majetich, J. O. Artman, M. DeGraef and S. W. Staley, Phys. Rev. B, 1994, 49, 11358 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: temperature dependence of magnetization for nanoparticles. See DOI: 10.1039/b614592b |
| This journal is © The Royal Society of Chemistry 2007 |
