Combining passive sampling and toxicity testing for evaluation of mixtures of polar organic chemicals in sewage treatment plant effluent †
Renee
Muller
*a,
Janet Y. M.
Tang
a,
Ricarda
Thier
b and
Jochen F.
Mueller
a
aNational Research Centre for Environmental Toxicology (EnTox), The University of Queensland, 39 Kessels Rd., Brisbane, QLD 4108, Australia. E-mail: r.muller@uq.edu.au; Fax: +61 7 3274 9003; Tel: +61 7 3274 9147
bSchool of Biomedical Sciences, The University of Queensland, St. Lucia, QLD 4068, Australia
First published on 10th November 2006
Abstract
Effluent from sewage treatment plants has been associated with a range of pollutant effects. Depending on the influent composition and treatment processes the effluent may contain a myriad of different chemicals which makes monitoring very complex. In this study we aimed to monitor relatively polar organic pollutant mixtures using a combination of passive sampling techniques and a set of biochemistry based assays covering acute bacterial toxicity (Microtox™), phytotoxicity (Max-I-PAM assay) and genotoxicity (umuC assay). The study showed that all of the assays were able to detect effects in the samples and allowed a comparison of the two plants as well as a comparison between the two sampling periods. Distinct improvements in water quality were observed in one of the plants as result of an upgrade to a UV disinfection system, which improved from 24× sample enrichment required to induce a 50% response in the Microtox™ assay to 84×, from 30× sample enrichment to induce a 50% reduction in photosynthetic yield to 125×, and the genotoxicity observed in the first sampling period was eliminated. Thus we propose that biochemical assay techniques in combination with time integrated passive sampling can substantially contribute to the monitoring of polar organic toxicants in STP effluents .
Introduction
Sewage treatment plant (STP) effluent is an important source for entry of anthropogenic organic chemical pollutants and their degradation products into the aquatic environment.1,2 Mixtures of a diverse range of potentially bioactive chemicals can be released, including pharmaceuticals, legal and illicit drugs, dietary supplements, active ingredients in personal care products, diagnostic agents, sun-screens, and numerous others.1,3 Treatment related processes within STPs such as phase partitioning (liquid to sludge) of more hydrophobic chemicals and production of hydrophilic (‘polar’) biodegradation products within STPs should lead to a proportional dominance of polar compounds in sewage effluent when compared with influent composition. Hence estimation of risk associated with polar chemicals in sewage effluent is important.Monitoring STP effluent is difficult due to matrix complexity and because the concentration and type of chemicals in the effluent may vary substantially within a small time frame depending on the composition and volume of incoming sewage and treatment efficiency.4 As such, reliance of monitoring programs on spot sampling provides limited environmentally relevant information and data require careful interpretation where the number of samples collected is inadequate.
The available methodology for assessment of organic pollutant mixture toxicity is often either inadequate or too labour intensive for routine monitoring applications. Sample chemical analysis for comparison with toxicity data for individual chemicals is a reasonably accessible method but frequently fails to account for all sample toxicity .2,5,6In vivo analysis of whole samples can be confounded by dominant ammonium toxicity unless extensive ‘toxicity identification evalution’ (TIE) procedures are followed.7 The method requires toxicants to be present at environmental levels which cause acute or short term biological effects as samples are tested in dilution series. Assessment of biomarkers such as vitellogenin or ethoxyresorufin-O-deethylase (EROD) activity induction is effective for determining toxicant induced changes in biological systems, but is labour intensive due to the required use of caged fish and rarely applied for routine monitoring.
Combining passive samplers for polar pollutants with high through-put, biochemical based assays has the potential to offer a simple and cost effective means to determine potential acute and chronic impacts of polar organic chemicals in sewage effluent . While assessment of toxicity of polar passive sampler extracts is relatively novel, the more established semi-permeable membrane device (SPMD)8,9 passive samplers for non-polar pollutants have been shown to provide a useful pre-concentration technique for bioassays .10–13 Highly concentrated extracts can be produced which are well suited for use in high throughput biological and biochemical testing, and the method has been identified as a potentially useful pre-concentration step for effect driven analysis (EDA).6
Polar organic passive samplers (POS) have been developed more recently by Kingston et al.14 (3M Empore Disks™ samplers, Chemcatcher) and Alvarez et al.15 (polar organic chemical integrated sampler, POCIS). Both utilise solid phase sorbents usually applied for laboratory sample extraction. 3M Empore Disks™ samplers allow some flexibility in chemical selectivity and uptake kinetics through application of different sampling matrices16 and addition of specific membranes.14 Stephens et al.16 calibrated styrene divenylbenzene (SDB-RPS) and C18 based Empore Disks™ exposed in a Teflon Chemcatcher type sampler housing14 without a membrane (‘naked’) and determined aqueous boundary layer mass transfer coefficients for several polar organic herbicides. Recently in our laboratory, Stephens et al. (publication in preparation) have undertaken field- and laboratory-based calibration studies utilizing different configurations of the sampler (deployment devices, use of membranes). One configuration of the sampler using SDB-RPS Empore Disks™ with a polysulfone ethoxylate membrane, in a Chemcatcher type sampler housing14 showed particular promise as a sampler for polar organic pollutants. The work demonstrated that this specific configuration allows relatively long term (>1 month) time integrated sampling for a wide range of polar organic pollutants.
To date, few studies are available combining passive samplers for polar organic chemicals with toxicity testing. Petty et al.17 and Vermeirssen et al.18 have tested extracts from POCIS using the YES assays for estrogenicity, and Escher et al.19 have recently published the first results combining ‘naked’ 3M Empore Disk™ passive samplers with the new Max-I-PAM phytotoxicity assay.
The objective of this study was to assess the biological effects of polar organic pollutants in STP effluent and to demonstrate the value of combining polar passive sampling with multiple biochemical assays. We present the first results of testing Empore Disk™ polar passive sampler extracts for both genotoxicity and bacterial acute toxicity and have also applied the same phytotoxicity assay reported by Escher et al.17 Results are discussed with reference to method efficacy, for inclusion in a comprehensive screening system for mixture toxicity of organic pollutants that employs passive samplers for both polar and non-polar pollutants and covers a wider range of toxicological endpoints.
Experimental
Sampling methodology
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3 of effluent per day and is a biological nutrient removal system producing secondary treated effluent (nutrient removal, no disinfection). Approximately 40% of waste received is trade waste (industrial, medical and commercial sources) and the balance consists of domestic waste. STP 2 produces secondary quality effluent through primary settlement and a trickling filter system with chlorine disinfection (no nutrient removal). Prior to the second month of sampling STP 2 was converted to a UV disinfection system. More than 90% of the 20
000 m3 of effluent per day and is a biological nutrient removal system producing secondary treated effluent (nutrient removal, no disinfection). Approximately 40% of waste received is trade waste (industrial, medical and commercial sources) and the balance consists of domestic waste. STP 2 produces secondary quality effluent through primary settlement and a trickling filter system with chlorine disinfection (no nutrient removal). Prior to the second month of sampling STP 2 was converted to a UV disinfection system. More than 90% of the 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3 treated per day by STP 2 is of domestic origin. Samplers at this site were deployed in the chlorine contact tank during month 1 and in the effluent stream prior to release during month 2.
000 m3 treated per day by STP 2 is of domestic origin. Samplers at this site were deployed in the chlorine contact tank during month 1 and in the effluent stream prior to release during month 2.
Empore Disks™ were prepared by conditioning in 100% methanol (HPLC grade, Merck). Samplers were transported in Chemcatcher sampling devices containing MilliQ water. Procedural blanks were produced for each site, consisting of unexposed disks contained within Teflon sampling devices which were stored at 4 °C for the duration of the sampling period.
The Empore Disk™ extraction procedure followed a method similar to that of Shaw and Müller.20 Disks were extracted twice in an ultrasonic bath for 5 min with 5 mL methanol. The two 5 mL volumes were combined and reduced in volume to approximately 1 mL before being filtered through a Millex 0.45 µM syringe driven filter unit. Extracts were transferred to glass vials and evaporated under a gentle stream of nitrogen to 250 µL, and then made up to exactly 1 mL with MilliQ water. Extracts from 2 samplers were combined prior to bioassay analysis.
Sampling rates may also vary depending on environmental conditions such as flow or turbulences affecting the POS sampler : water boundary layer, temperature and biofouling. These have not been accounted for in the present study as limited methods are presently available for in situ calibration of these devices. Variation due to flow is likely to be the greatest source of sampling rate variability and this has been minimized through the design of the sampling device which promotes an increased boundary layer.
Dose response assessments
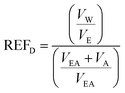 | (1) |
 | (2) |
The marine diatom Phaeodactylum tricornutum was used for phytotoxicity assessment. Algae were grown in a culture chamber at 23 °C with a 12 h natural dark/light cycle receiving 50 µE m–2 s–1. Log phase cultures were used for assays, by subculturing algae 3 days prior to analysis. For a detailed description of growth conditions and media see Bengtson Nash et al.24 Testing was undertaken using a measuring light intensity of 10 and measuring frequency of 8 (3 µmol quanta m–2 s–1 photosynthetically active radiation (PAR), no actinic light). Five readings were averaged after 1 h incubation. A full description of the methodology is published elsewhere.27 Samples and procedural blanks were tested at twelve concentrations (maximum REFD 530) in duplicate. A diuron positive control was also tested at 16 concentrations (0.07–34 µg L–1). Concentration–effect curves were fitted using eqn (2), minimum (0%) and maximum (100%) effect values and slope (s = 1) were fixed to allow comparison of EC(REFD)50 values between samples and positive control.
Diuron equivalent concentrations (DEC) represent the concentration of diuron (µg L–1) which would be required to produce an effect equal to that observed in samples.19,23 These were calculated from EC50 values as per eqn (3).
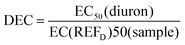 | (3) |
Results
Bacterial acute toxicity
All samples showed toxicity in the Microtox™ bioassay (EC(REFD)50 values are provided in Table 1), though results indicated only minimal acute toxicity due to organic chemical contaminants would occur at environmental levels (REFD = 1) (Fig. 1). STP 2 produced the most toxic sample during the first month of sampling; following the STP 2 plant upgrade this toxicity was reduced below levels observed in samples collected from STP 1. Responses observed at STP 1 were highly consistent between sampling periods. Some toxicity was observed in procedural blanks, up to about 30% toxicity at the highest enrichment level.| Microtox | Max-I-PAM | umuC | ||||
|---|---|---|---|---|---|---|
| EC(REFD)50 | 95% CI | EC(REFD)50 | 95% CI | LOEC(REFD) (–S9) | LOEC(REFD) (+S9) | |
| a NR: no response in bioassay . b No dose response analysis was undertaken, 4-NQO tested at 50 ng mL–1 and 2-AA at 200 ng mL–1. | ||||||
| STP 1 | ||||||
| Month 1 | 64 | (60–69) | 24 | (20–29) | NR | NR |
| Month 2 | 68 | (63–74) | 35 | (31–42) | 119 | 119 |
| STP 2 | ||||||
| Month 1 | 26 | (23–29) | 30 | (117–135) | 33 | NR |
| Month 2 | 84 | (74–95) | 125 | (117–135) | NR | NR |
| Positive control | 17 mg l–1 (phenol) | (13–22) mg l–1 | 6.7 µg L–1 (diuron) | (6.1–7.4) µg L–1 | Positiveb (4-NQO) | Positiveb (2-AA) |
| Blank | 1500 | (920–2500) | NR | — | NR | — |
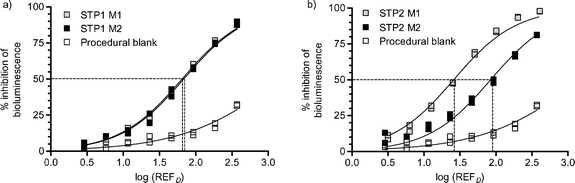 | ||
| Fig. 1 Concentration–effect curves for Microtox™ analysis at (a) STP 1 and (b) STP 2 during months 1 and 2 sampling (M1 and M2, respectively). Duplicate results plotted. R2 values for concentration–effect curves > 0.9870. EC(REFD)50 values are listed in Table 1. | ||
 | ||
| Fig. 2 Concentration–effect curves for Max-I-PAM analysis, inhibition of PSII photosynthetic yield at (a) STP 1 and (b) STP 2 during months 1 and 2 sampling (M1 and M2, respectively). Duplicate results plotted. R2 values for concentration–effect curves > 0.9577. EC(REFD)50 values are listed in Table 1. | ||
Phytotoxicity
Phytotoxicity was relatively consistent between months 1 and 2 of sampling at STP 1 and month 1 of sampling at STP 2, where diuron equivalent concentrations (DEC) of 0.28, 0.19 and 0.22 µg L–1, respectively, could be calculated from bioassay results. Following the upgrade of STP 2, a DEC of 0.05 µg L–1 was recorded. No response was observed in procedural blanks tested.Genotoxicity
Genotoxicity was observed in samples from both treatment plant in the absence of metabolic activation (S9 mix). At STP 1, 100× sample enrichment was required to produce a positive response. Where chlorination treatment was employed at STP 2, a genotoxic response was exhibited in samples enriched up to 30×, this effect was not present in samples collected following the plant upgrade. Table 1 displays LOEC(REFD) values for samples tested without metabolic activation. Genotoxicity figures are provided in the ESI.†Testing of samples in the presence of an S9 mix is expected to result in an increase in genotoxicity due to metabolic activation of progenotoxins. Our results indicated the opposite response and genotoxicity was reduced in all samples (Table 1). Similar results have been observed by others and it has been suggested that sorption of genotoxins to proteins in the S9 mix results in the non-expression of the mutagenicity of some direct and indirect mutagens .23,24 The observed reduction in genotoxicity in the presence of S9 in samples tested in this study cannot therefore be considered to have implications for sample genotoxicity.
Discussion
The results from the bioassays suggest that different biological effects may be expected as a result of organic chemical pollution in the receiving zones of the two treatment plants. Fig. 3 suggests phytotoxicity is the most toxicologically relevant endpoint (of those tested) at STP 1. At STP 2, bacterial cytotoxicity and genotoxicity were potential endpoints of concern whilst chlorination disinfection was employed. Following conversion of STP 2 to UV treatment, all assays exhibited lowered toxicity .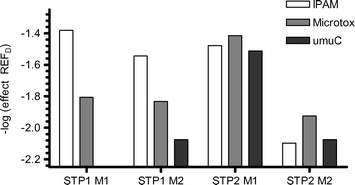 | ||
| Fig. 3 Comparison of sample enrichment required to produce a defined effect in each assay (Microtox: EC(REFD)50; Max-I-PAM: EC(REFD)50; umuC: LOEC(REFD). Values available in Table 1. | ||
The effluent undergoing chlorination disinfection exhibited higher levels of both bacterial cytotoxicity and genotoxicity when compared with both UV treated effluent from the same plant and effluent from STP 1 (no disinfection). As chlorine is not expected to accumulate in POS, elevated cytotoxicity and genotoxicity responses in chlorine treated effluent can be tentatively attributed to chlorination byproduct toxicity , as has been observed previously in chlorine treated effluent ,30,31 however further data are required to support this hypothesis.
The results of the three bioanalytical endpoints employed in this study indicate that a substantial improvement in effluent quality was achieved through the upgrade of STP 2. Such data provides useful information to guide both ecological and, where wastewater is intended for agricultural or potable water reuse, human health risk assessment and can be used to guide management initiatives.
Method efficacy for aquatic pollutant risk assessment
The limitations of conventional monitoring approaches have been widely recognized. For example, Eggen et al.32 have listed among the current challenges in ecotoxicology: low concentrations of pollutants and long exposure times (chronic effects), multiple effects by single pollutants, and complex mixtures of pollutants. Daughton and Ternes1 have also highlighted potential long term (multigenerational) impacts which may go undetected by the use of conventional risk assessment techniques. In the present study we have shown that combining passive sampling for polar organic pollutants with bioassays can provide a sensitive and relatively simple method for detecting effects of organic chemical pollutants across acute and chronic endpoints.Passive sampling for polar pollutants is an attractive option in sewage effluent monitoring, allowing a time weighted average concentration of pollutants across the sampling period to be obtained and minimizing the need to handle effluent samples. The method enables extraction of larger volumes of water (up to several litres), required for detection of hormones and other pharmaceuticals which are potentially bioactive at trace levels (nanogram per litre). While use of passive samplers for non-polar pollutants is well established, polar sampling devices have been developed more recently. Further developmental and calibration work with these types of samplers is required to characterize uptake rates for a greater range of environmental pollutants and difficulties remain with regards to in situ calibration of the devices (correction for changes in uptake rates relating to flow, temperature, biofouling etc. As calibration data become available for a wider range of chemicals, sampling rates for relevant chemicals may be applied to different bioassays .
Consistency between results obtained in months 1 and 2 of sampling at STP 1 (Fig. 1 and 2), supports the advantages of implementing a time integrated sampling approach. Chemical concentrations in sewage effluent can be expected to vary substantially depending on the time of day and week, and the ability to collect time integrated samples improves the environmental relevance of results by more closely representing concentrations to which organisms are exposed in the effluent receiving zone.
To assess potential biological impacts of polar organic chemicals in sewage effluent , we have applied low volume, biochemical based assays. This approach precludes environmental realism as whole organisms and ecosystems can be expected to react differently than might be predicted from a ‘test tube’ based approach. Thus the method does not replace traditional ecotoxicological risk assessment but should instead be applied to complement available techniques and can be considered a bio-recognition system for detection of pollutants through toxic effects. In this capacity, the use of passive samplers for in situpreconcnetration shows promise for inclusion in effects driven analysis (EDA) studies. Use of passive samplers for both polar and non-polar organic pollutants samplers will enable in situ sample fractionation , and use of an increased battery of assays (including additional endpoints such as estrogenicity, cellular detoxification mechanisms, or neurotoxicological endpoints such as acetylcholine esteraseinhibition ) will provide a powerful method for assessing effects of site specific mixtures of organic pollutants.
Use of enriched extracts allows assessment of effects even where chemicals are present in source water at concentrations well below those that would cause environmental harm. Such sensitivity will allow preventative management initiatives and may prove vital for assessment of effects of trace contaminants in re-use water.
Conclusions
Combining time integrated passive sampling with a battery of high through-put bioassays allows us to undertake meaningful assessment of organic chemical pollutants in complex exposure environments. The method shows promise as an effective means to characterize sample toxicity in STP effluent and in this study important endpoints of concern have been identified. Further field and laboratory testing is required to determine the extent of method sensitivity in more pristine environments and at sites subjected to other sources of contamination.Acknowledgements
The contributions of Dr Ulrich Schreiber in development of the Max-I-PAM instrument and methodology as well as comments on the manuscript are gratefully acknowledged. The laboratory assistance of Pamela Quayle and Kristie Lee Chue is appreciated. Thanks also to Dr Frederick Leusch for comments on the manuscript. This project is funded by an Australian Research Council Linkage Grant, with industry partners including: Queensland EPA, New South Wales DEH, Victoria EPA, SA Water, Brisbane Water, SEQ Water, Queensland Health, Australian Plague Locust Commission, Derwent Estuary Program and Environment Agency UK. Queensland Health provides funding for the National Research Centre for Environmental Toxicology.References
- C. G. Daughton and T. A. Ternes, Environ. Health Perspect., 1999, 107, 907–938 CrossRef CAS.
- J. Garric, B. Vollat, D. K. Nguyen, M. Bray, B. Migeon and A. Kosmala, Water Sci. Technol., 1996, 33, 83–91 CrossRef CAS.
- B. Halling-Sorensen, S. Nors Nielsen, P. F. Lanzky, F. Ingerslev, H. C. Holten Liitzhofl and S. E. Jorgensen, Chemosphere, 1998, 36, 357–393 CrossRef CAS.
- N. M. Vieno, T. Tuhkanen and L. Kronberg, Environ. Sci. Technol., 2005, 39, 8220–8226 CrossRef CAS.
- K. C. Donnelly, R. Lingenfelter, L. Cizmas, M. H. Falahatpisheh, Y. C. Qian, Y. Tang, S. Garcia, K. Ramos, E. Tiffany-Castiglioni and M. M. Mumtaz, Environ. Toxicol. Pharmacol., 2004, 18, 135–141 CrossRef CAS.
- W. Brack, Anal. Bioanal. Chem., 2003, 377, 397–407 CrossRef CAS.
- R. M. Burgess, K. T. Ho, M. D. Tagliabue, A. Kuhn, R. Comeleo, P. Comeleo, G. Modica and G. E. Morrison, Mar. Pollut. Bull., 1995, 30, 524–535 CrossRef CAS.
- J. N. Huckins, M. W. Tubergen and G. K. Manuweera, Chemosphere, 1990, 20, 533–552 CrossRef CAS.
- J. N. Huckins, G. K. Manuweera, J. D. Petty, D. Mackay and J. A. Lebo, Environ. Sci. Technol., 1993, 27, 2489–2496 CAS.
- J. N. Huckins, J. D. Petty, J. A. Lebo, C. E. Orazio, H. F. Prest, D. E. Tillitt, G. S. Ellis, B. T. Johnson and G. K. Manuweera, in Techniques in Aquatic Toxicology, ed. G. K. Ostrander, CRC-Lewis Publishers, Boca Raton, FL, 1996, pp. 625–655 Search PubMed.
- R. Muller, R. Thier, J. Tang and J. F. Mueller, Assessing STP effluent mixture toxicity using passive samplers and four bioassays, in ‘Contaminants of Concern in Water’ Conference Proceedings, June 22–23, 2005, Australian Water Association, Brisbane Search PubMed.
- J. D. Petty, S. B. Jones, J. N. Huckins, W. L. Cranor, J. T. Parris, T. B. McTague and T. P. Boyle, Chemosphere, 2000, 41, 311–321 CrossRef CAS.
- D. Sabaliunas and A. Sodergren, Environ. Pollut., 1997, 96, 195–205 CrossRef CAS.
- J. K. Kingston, R. Greenwood, G. A. Mills, G. M. Morrison and L. B. Persson, J. Environ. Monit., 2000, 2, 487–495 RSC.
- D. A. Alvarez, J. D. Petty, J. N. Huckins, T. L. Jones-Lepp, D. T. Getting, J. P. Goddard and S. E. Manahan, Environ. Toxicol. Chem., 2004, 23, 1640–1648 CrossRef CAS.
- B. S. Stephens, A. Kapernick, G. Eaglesham and J. F. Mueller, Environ. Sci. Technol., 2005, 39, 8891–8897 CrossRef CAS.
- J. D. Petty, J. N. Huckins, D. A. Alvarez, W. G. Brumbaugh, W. L. Cranor, R. W. Gale, A. C. Rastall, T. L. Jones-Lepp, T. J. Leiker, C. E. Rostad and E. T. Furlong, Chemosphere, 2004, 54, 695–705 CrossRef CAS.
- E. L. M. Vermeirssen, O. Korner, R. Schonenberger, M. J.-F. Sutter and P. Burkhardt-Holm, Environ. Sci. Technol., 2005, 39, 8191–8198 CrossRef CAS.
- B. I. Escher, P. A. Quayle, R. Muller, U. Schreiber and J. F. Mueller, J. Environ. Monit., 2006, 456–464 RSC.
- M. Shaw and J. F. Müller, Mar. Pollut. Bull., 2005, 51, 876–881 CrossRef CAS.
- AZUR Environmental, Microtox AZUR Test Manual: Basic Test for Aqueous Extract, http://www.azurenv.com/mtox.htm, accessed March 2005 Search PubMed.
- U. Schreiber, J. F. Mueller, A. Haugg and R. Gademann, Photosynth. Res., 2002, 74, 317–330 CrossRef CAS.
- S. M. Bengtson Nash, K. McMahon, G. Eaglesham and J. F. Muller, Mar. Pollut. Bull., 2005, 51, 351–360 CrossRef CAS.
- S. M. Bengtson Nash, U. Schreiber, P. J. Ralph and J. F. Muller, Biosens. Bioelectron., 2004, 20, 1443–1451.
- B. I. Escher, N. Bramaz, R. I. L. Eggen and M. Richter, Environ. Sci. Technol., 2005, 39, 3090–3100 CrossRef CAS.
- U. Schreiber, in Chlorophyll Fluorescence. A Signature of Photosynthesis, ed. G. Papageorgiou and Govindjee, Kluwer Academic Publishers, Dordrecht, The Netherlands, 2004 Search PubMed.
- U. Schreiber, P. Quayle, S. Schmidt and J. F. Mueller, Biosens. Bioelectron., 2006 DOI:10.1016/j.bios.2006.10.018.
- J. H. Miller, in Experiments in Molecular Genetics, Cold Spring Harbor Laboratory Press, NY, 1972, pp. 431–433 Search PubMed.
- Y. Oda, S. Nakamura, I. Oki, T. Kato and H. Shinagawa, Mutat. Res., 1985, 147, 219–229 CAS.
- S. Monarca, D. Feretti, C. Collivignarelli, L. Guzzella, I. Zerbini, G. Bertanza and R. Pedrazzani, Water Res., 2000, 34, 4261–4269 CrossRef CAS.
- I. Blatchley, R. Ernest, B. A. Hunt, R. Duggirala, J. E. Thompson, J. Zhao, T. Halaby, R. L. Cowger, C. M. Straub and J. E. Alleman, Water Res., 1997, 31, 1581–1588 CrossRef.
- R. I. L. Eggen, R. Behra, P. Burkhardt-Holm, B. I. Escher and N. Schweigert, Environ. Sci. Technol., 2004, 31, 59A–64A.
Footnote |
| † Electronic supplementary information (ESI) available: Figures displaying genotoxicity response and cytotoxicity in the umuC assay. See DOI: 10.1039/b612430e |
| This journal is © The Royal Society of Chemistry 2007 |
