Molecular dynamics simulations were used to study the evaporation from water clusters containing either Cl−, H2PO4−, Na+ or NH4+ ions. The simulations ranged between 10 and 500 ns, and were performed in vacuum starting at 275 K. A number of different models were used including polarizable models. The clusters contain 216 or 512 molecules, 0, 4 or 8 of which were ions. The ions with hydrogen bonding properties do not affect evaporation, even though the phosphate ions have a pronounced ion–ion structure and tend to be inside the cluster whereas ammonium shows little ion–ion structure and has a distribution within the cluster similar to that of the water molecules. Since the individual ion–water interactions are much stronger in the case of Na+–water and Cl−–water clusters, evaporation is somewhat slower for clusters containing these ions. It seems therefore that the main determinant of the evaporation rate in ion–water clusters is the strength of the interaction. Fission of droplets that contain more ions than allowed according to the Rayleigh limit seems to occur more rapidly in clusters containing ammonium and sodium ions.
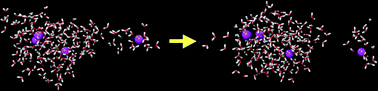
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
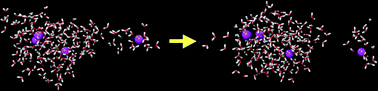

 Please wait while we load your content...
Please wait while we load your content...