Mechanical stimuli-driven cancer therapeutics
Jusung
An†
a,
Hyunsik
Hong†
b,
Miae
Won
a,
Hyeonji
Rha
a,
Qihang
Ding
a,
Nayeon
Kang
b,
Heemin
Kang
 *b and
Jong Seung
Kim
*b and
Jong Seung
Kim
 *a
*a
aDepartment of Chemistry, Korea University, Seoul 02841, Korea. E-mail: jongskim@korea.ac.kr
bDepartment of Materials Science and Engineering, Korea University, Seoul 02841, Korea. E-mail: heeminkang@korea.ac.kr
First published on 13th December 2022
Abstract
Mechanical stimulation utilizing deep tissue-penetrating and focusable energy sources, such as ultrasound and magnetic fields, is regarded as an emerging patient-friendly and effective therapeutic strategy to overcome the limitations of conventional cancer therapies based on fundamental external stimuli such as light, heat, electricity, radiation, or microwaves. Recent efforts have suggested that mechanical stimuli-driven cancer therapy (henceforth referred to as “mechanical cancer therapy”) could provide a direct therapeutic effect and intelligent control to augment other anti-cancer systems as a synergistic combinational cancer treatment. This review article highlights the latest advances in mechanical cancer therapy to present a novel perspective on the fundamental principles of ultrasound- and magnetic field-mediated mechanical forces, including compression, tension, shear force, and torque, that can be generated in a cellular microenvironment using mechanical stimuli-activated functional materials. Additionally, this article will shed light on mechanical cancer therapy and inspire future research to pursue the development of ultrasound- and magnetic-field-activated materials and their applications in this field.
 Nayeon Kang | Nayeon Kang received her BS degree in Materials Science and Engineering from Korea University, Seoul, in 2021. She is pursuing her PhD degree in Prof. Heemin Kang's laboratory since 2021. |
Key learning points1. General significance of mechanical stimuli in cancer therapy.2. Fundamental mechanism of mechanical stimuli-driven cellular signaling. 3. The clinical advantages of mechanical stimuli-driven cancer therapy mode using tissue-penetrative ultrasound and magnetic fields. 4. The strategies for enhancing mechanical stimuli-driven cancer therapeutic efficacy. 5. Recent development, challenges, and perspectives for mechanical stimuli-driven cancer therapy strategies. |
1. Introduction
External energy source-mediated cancer therapy has drawn considerable attention due to its paramount significance in enhancing treatment efficacy. Indeed, therapies involving various fundamental stimuli, including light in phototherapy (photodynamic therapy [PDT] and photothermal therapy [PTT]),1,2 heat in thermal therapy,3 electricity in electrodynamic therapy,4 radiation in X-ray/Cherenkov PDT,5 and microwaves in microwave dynamic/thermal therapy,6 have been established in basic research or preclinical fields as novel cancer therapeutic strategies. Nevertheless, many clinical situations require the limitations of these therapies to be overcome such as (1) tissue scattering, (2) poor tissue penetration, and (3) low targeting capabilities. Hence, strategies involving “mechanical stimuli” that utilizes deep tissue-penetrating and focusable energy sources (e.g., ultrasound and magnetic fields) have emerged as promising cancer treatment modalities that provide patient-friendly and effective therapeutic strategies while overcoming the shortcomings of conventional cancer therapies based on fundamental stimuli.7,8 Continuous efforts have recently demonstrated versatile clinical utility using low intensity ultrasound and magnetic fields for cancer treatment. This application has been investigated extensively and determined to be highly effective in treating various target cancers under physical impact based on “mechanical force” which differs from the previous discussion of ultrasound- and magnetic-stimuli therapy that had been reported.8–14The fundamental mechanical forces are intrinsically present throughout the cellular microenvironment due to normal motion and physiological functioning of cells and sub-organelles.15 For several decades, studies have revealed that mechanical forces drive numerous physiological processes and are a crucial regulator of cellular interaction.16,17 Conversely, a cytotoxic factor can be produced within the cell environment when the intensity of exogenous mechanically forced stimulation achieves an excessive level that can directly damage the cell.7,8,17 This level of mechanical force can contribute to creating appropriate conditions for enhanced cancer therapy by generating a structural change that can directly affect cancer cells.17 Thus, the last decade has witnessed tremendous progress regarding the use of “mechanical stimuli” in the cancer treatment field based on a new angle of utilizing ultrasound and magnetic fields. Most recently, as Ardem Patapoutian won the Nobel Prize in Physiology or Medicine in 2021, it is valid to consider the established theory that mechanical activation directly affects the biological environment.18 The mechanical cancer therapy driven by ultrasound and magnetic fields is based on the fundamental mechanical forces with the subclassification of compression, tension, shear force, and torque that are ubiquitously achieved in a cellular microenvironment or easily applied externally. To better understand the role of microscopic mechanical stress in the context of cellular morphogenesis caused by ultrasound and magnetic fields, the physical forces that can directly affect cells are described below.
(1) “Compression” is a pressing force that can physically provide external pressure on plasma membranes or sub-organelles. (2) “Tension” is a pulling force that causes coercive expansion of the cell structure. (3) “Shear force” is associated with the amputation of a cellular organism that results in cell structures pushing each other in opposite directions. (4) “Torque” is a force that induces twisting and pinching stimulation toward cellular membranes or sub-organelles. Such forces can be exerted on cells through the various synchronized physical motions of the mechanical stimuli-activated functional materials, and consequently, these mechanical stimulations can exert a direct anti-cancer therapeutic effect by inducing cell structural collapse and thus cellular damage. Ultrasound and magnetic fields can generate mechanical forces on cell structures such as the plasma membrane surface or internal sub-organelles (e.g., mitochondria, lysosomes, or nuclei) that induce coercive morphological changes and intracellular damage. Furthermore, it can provide indirect support to enhance other treatment systems such as chemotherapy, immunotherapy, gene therapy, gas therapy, PDT, and sonodynamic therapy (SDT) as combinational therapeutic strategies. Hence, mechanical cancer therapy achieved by utilizing ultrasound or magnetic fields represents a promising modality that offers effective cancer treatment through the simple application of versatile mechanical stimulation (Scheme 1).
 | ||
| Scheme 1 Mechanical stimuli-driven cancer therapeutics mediated by various mechanical forces (compression, tension, shear force, and torque) via the application of ultrasound and magnetic fields. | ||
This tutorial review describes the fundamental principles of the assorted types of mechanical forces induced by ultrasound- and magnetic field-driven stimuli and their interactions with target sub-organelles in microscopic cellular environments. Additionally, various therapeutic strategies for cancer therapy are summarized in addition to mechanical stimulation using ultrasound and magnetic fields. We will discuss novel functional materials, their characterization, and their biological applications, although it should be noted that these still need further consideration for real clinical applications (Table 1).
| Energy sources | Mechanical forces | Nano/micro materials | Force-generating mechanism | Irradiation modes | Therapeutic application | Ref. |
|---|---|---|---|---|---|---|
| Abbreviations: NPs, nanoparticles; FU, focused ultrasound; DC, duty cycle; MB, microbubble; US, ultrasound; TSP, temperature-sensitive plateletsome; HIFU, high-intensity focused ultrasound; siRNA, small interfering ribonucleic acid; HMPB, hollow mesoporous Prussian blue; PFH, perfluorohexane; tHSA, thiolated human serum albumin; PLGA, poly(lactic-co-gycolic acid); LPO, lipid peroxidation; MOF, metal–organic framework; MNPs, magnetic nanoparticles; SMF, static magnetic field; SPIONs, superparamagnetic iron oxide nanoparticles; PMF, pulsed magnetic field; RMF, rotating magnetic field; AMF, alternating magnetic field; HAFP, hyaluronic acid, protamine, and ferumoxytol; MNM, magnetic nanomotor; IONs, iron oxide nanocubes; USPIONs, ultra-small superparamagnetic iron oxide nanoparticles; SMNPs, superparamagnetic nanoparticles; DMF, dynamic magnetic field. | ||||||
| Ultrasound | Compression + Shear force | Liposomal NPs | Sonoporation | FU (3 W cm−2, 1 MHz, 10% DC) | Neuroblastoma | 30 |
| MB-NPs | Sonoporation | US (4 W cm−2, 1 MHz, 50% DC) | Breast cancer (4T1) | 31 | ||
| Spherical siRNA-NPs | Sonoporation | FU (10 W cm−2, 1.5 MHz, 5% DC) | Melanoma (B16F10), carcinoma (SCC-7) | 32 | ||
| Tension | TSP | MB implosion | HIFU (0–200 W, 0.6–1.8 MHz) | Carcinoma (HeLa) | 34 | |
| HMPBs-PFH | MB implosion | HIFU (120 W, 5–12 MHz) | Breast cancer (MDA-MB-231) | 35 | ||
| Spherical tHSA-NPs-MB | MB implosion | HIFU (10 W cm−2, 1.5 MHz, 5% DC) | Lung cancer (A549) | 36 | ||
| Fe3O4-PFH/PLGA | MB implosion | HIFU (0.8 MHz) | Carcinoma (VX2) | 37 | ||
| Shear force | Liposome | LPO | US (1 W cm−2, 1 MHz, 50% DC) | Breast cancer (4T1) | 45 | |
| Liposome | LPO | US (0.35 W cm−2, 1 MHz) | Breast cancer (4T1) | 46 | ||
| Ga-Fe(II)@liposome | LPO | US (1.5 W cm−2, 1 MHz, 50% DC) | Breast cancer (MCF-7/ADR) | 47 | ||
| Mn-MOF | LPO | US (0.9 W cm−2, 1 MHz, 30% DC) | Breast cancer (4T1) | 48 | ||
| Magnetic field | Compression + Tension | Spherical MNPs (Zn0.4Fe2.6O4) | Directional MNP movement | SMF (0.2 T) | Colon cancer (DLD-1) | 59 |
| Spherical SPIONs (Fe3O4) | Pulsed SPION movement | PMF (5–8 T, 10 pulses, 10 s interval) | Liver cancer (Huh7, Alexander, and HepG2) | 61 | ||
| Sharp MMP (Fe3O4) | MMP vibration | RMF (0.6 T, 20 Hz) | Glioblastoma (U87-MG) | 69 | ||
| Spherical MNPs (Fe) | MNP vibration | OMF (0.2 T, 10 Hz) | Liver cancer (HepG2) | 76 | ||
| Spherical HAPF | HAPF vibration | AMF (1.5 mT, 1 Hz) | Natural killer cell | 77 | ||
| Spherical MNPs (Zn0.4Fe2.6O4) | Directional MNP movement | SMF (0.5 T) | Drug-resistant colon cancer (DLD-1/ADR) | 78 | ||
| Shear force | Rod-like MNPs (Zn0.4Fe2.6O4) | MNP rotation | RMF (40 mT, 15 Hz) | Glioblastoma cell mitochondria (U87), liver cancer cell mitochondria (HepG2) | 71 | |
| Rod-like FDP (Fe3O4@Dex-PGEA) | FDP rotation | AMF | Breast cancer (4T1) | 80 | ||
| Rod-like MNM (Zn0.4Fe2.6O4) | MNM rotation | RMF (40 mT, 15 Hz) | Glioblastoma (U87), breast cancer (MDA-MB-231) | 81 | ||
| Rod-like IONs (Zn0.4Fe2.6O4) | ION rotation | RMF (40 mT, 15 Hz) | Glioblastoma (U87) | 82 | ||
| Torque | Microdisc (FeNi) | Microdisc spin | AMF (9 mT, 10–20 Hz) | Glioma (N10) | 54 | |
| Spherical MNPs (Fe3O4) | MNP spin | AMF (50 mT, 233 kHz) | Breast cancer (MDA-MB-231) | 63 | ||
| Spherical USPIONs (Fe3O4) | USPION rotation | RMF (40 mT, 1 Hz) | Pancreatic cancer-associated fibroblast lysosome (CAF-CCK2) | 64 | ||
| Spherical SMNPs (Fe3O4) | SMNP spin | AMF (125 mT, 50 Hz) | Breast cancer (MDA-MB-231, and BT474) | 66 | ||
| Spherical USPIONs (Fe3O4) | USPION rotation | RMF (40 mT, 1 Hz) | Pancreatic cancer-associated fibroblast lysosome (CAF-CCK2) | 74 | ||
| Spherical SPIONs (Fe3O4) | SPION spin | DMF (30 mT, 20 Hz) | Insulinoma cell lysosome (INS-1) | 84 | ||
| Nanospindle (Fe3O4) | Nanospindle spin | AMF (1 mT, 0.5–20.0 Hz) | Hepatoma cell mitochondria (McA-RH7777) | 85 | ||
2. Ultrasound
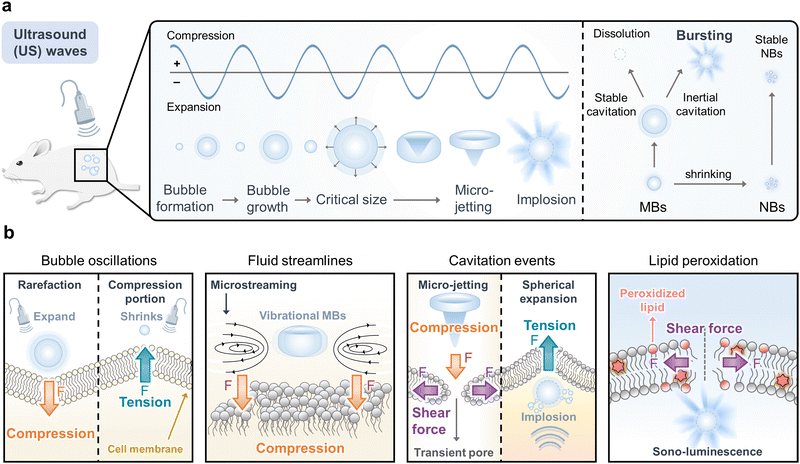
Ultrasound has been widely used in diagnostic and therapeutic applications due to its non-invasive modality and deep tissue-penetrating ability.7 It is a cost-effective and safe clinical tool that has been used for physiotherapy, ultrasonography, lithotripsy, and tumor ablation in high-intensity focused ultrasound (HIFU) over the last several decades.19 The thermal effect has been considered a primary factor in ultrasound-mediated cancer therapy, particularly with HIFU, and current research suggests various applications of and advancements in biomedical-focused ultrasound (FU) in the context of medicine such as SDT, drug delivery systems (DDS), or gene delivery systems based on cavitation pressure-induced mechanical stimulation. Thus, this session will provide a recent review regarding the development of “ultrasound-mediated mechanical cancer therapy.”The ultrasound-driven mechanical forces that arise from cavitation are related primarily to bubbles that are exogenously administered or created during the rarefactional cycle of acoustic pressure.20–23 The gas in a solution of body tissues is converted into microbubbles (MBs) through negative pressure or the administration of stabilized gas-filled MBs or nanobubbles (NBs) in factitial biomaterials.24 The bubbles then oscillate, burst, and impact nearby cellular building blocks such as cell membranes or sub-organelles.25 Hence, ultrasound-mediated mechanical cancer therapy with low-frequency insonation in the cancer region should be considered a practical cancer therapeutic tool in the clinical field, and many other versatile approaches have been suggested according to significant research.
2.1 Principle of acoustic cavitation
The physics of the acoustic cavitation (ultrasonic cavitation) process is associated with bubble formation (or administration), growth, and collapse in response to a pressure pulse. An explanation for cavitation bubble formation could be that when an ultrasound wave occurs in a liquid, the molecular structure undergoes alternating expansion and compression cycles in which molecules are pulled apart by rarefaction and pushed together by compression.21 As long as the ultrasound wave is sufficiently strong, it can overcome intermolecular binding forces and cause a sudden drop in pressure with the formation of gaseous bubbles (nuclei) in the liquid. Ultrasound waves allow these nuclei to grow with the ensuing cavitation cycles until they reach an unstable size (critical size) and then collapse violently (Fig. 1a). According to a developed crevice model of nucleation in an aqueous medium, cavitation bubbles typically form between nano and micron sizes (NBs and MBs, respectively) in fluids depending upon the frequency, power, and microenvironment.22 Besides, in an alternative way, stabilized gas-filled nanoparticles, which are factitial MBs or NBs biomaterials, can be exogenously delivered to act as cavitation bubbles for oscillating under ultrasound wave.24 Upon forming MBs on target location, the size grows until they reach a critical size known as their resonance size which is dependent upon the applied sound frequency. Eventually, the instability of MBs at the resonance size violently causes collapse and implosion within a small number of acoustic cycles, and this is termed transient cavitation.23 In contrast, stable cavitation (or non-inertial cavitation) occurs when the MBs oscillate for many cycles at or around their resonance size instead of violently collapsing. During stable cavitation, the ultrasound pressure drives bubbles to dissolve or shrink as they move past each other (Fig. 1a).24It should be emphasized that ultrasound-mediated mechanical stimulation is associated with acoustic cavitation bubble collapse close to or on a solid cellular structure. The solid surfaces provide resistance to liquid flow above a given pressure threshold, and bubble implosion occurs beyond a certain resonance size. Resultantly, the bubbles collapse asymmetrically,21 which produces a high-speed liquid jet (micro-jetting) that moves at a speed as fast as 111 m s−1 on the cellular structure.25,26 Hence, severe damages can be inflicted on the impact zones and surface pitting (erosion) can occur on the surface. Moreover, the local environment of a plasma membrane is influenced by acoustic streaming, and this is called microstreaming (fluid streamlines).23,24 Due to the pressure differences, microstreaming is generated around the MBs as they undergo vibrational motions, ultimately driving the surrounding fluid to flow around it in several patterns.24 Consequently, microstreaming could physically interfere with the interaction of the nearby lipid membranes. Both micro-jetting and microstreaming contribute to weakening the robustness of the cell membrane, and this induces “compression” and “shear forces.” Another fundamental physiological property of acoustic cavitation is sonoluminescence which refers to light production during insonation.24–26 Although the exact mechanism underlying light production in sonoluminescence is still unclear, it has been suggested that blackbody, bremsstrahlung, or recombination radiation, alone or in combination, may result in sonoluminescence. A sonosensitizer can be excited by sonoluminescence to generate electron–hole (e−–h+) pairs or triplet states with subsequent generation of reactive oxygen species (ROS) or radical species (˙OH) that react with endogenous substrates,27,28 particularly lipid layers as a lipid peroxidation (LPO), which can induce “shear force.”
Consequentially, these ultrasound-induced mechanical stimuli yield inertial cavitation, where inward rushing of fluid against unstable and expanding bubbles causes micro-jetting, bubble collapse, and implosion. Eventually, insonated cavitation results in energy release in the form of a shockwave yielding transient sonochemical hotspots (up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 K) that correspond to a liquid layer, high pressures (81 MPa), and mechanical forces. An ultrasound-mediated mechanical stimuli can generate the following forces.
000 K) that correspond to a liquid layer, high pressures (81 MPa), and mechanical forces. An ultrasound-mediated mechanical stimuli can generate the following forces.
(1) Bubble oscillations: sono-compression and tension caused by implosion or rapid shrinkage of MBs on the exterior plasma membrane. (2) Fluid streamlines: sono-compression induced by microstreaming tears the lipid layer apart from the vibration MBs outside the plasma membrane. (3) Cavitation events: sono-compression and shear force generated by micro-jetting or sono-tension induced by pulsatile cell swelling with HIFU. (4) LPO: induced shear force generated by peroxidation of the lipid membrane structure. These mechanical forces not only directly damage cancer cells to induce apoptotic or non-apoptotic cellular death but also induce indirect therapeutic mediation by enhancing other anti-cancer treatment systems (Fig. 1b).
2.2 Sono-compression- and shear force-mediated cancer therapy
Cavitation of MBs can exert biological and mechanical impacts on the cellular environment that would be valuable for medication administration while minimizing the danger of producing heat that can lead to tumor thermal ablation as a side effect of ultrasound. The oscillation of MBs on cell membranes caused by ultrasound induces “sonoporation” triggering from compression and shear force-based mechanical perturbation in the vicinity of bubbles to form transient microscopic pores (Fig. 2a).29 Sonoporation is known to inhibit tumor proliferation directly.29 Besides, MBs can be synergistically combined with ultrasound to cause gaps in the blood–brain barrier (BBB) that can aid in facilitating drug delivery into the brain, a process that is currently difficult. These mechanical effects can boost drug microvessel permeability, drug penetration across the interstitial space, and drug absorption, thereby improving anti-cancer effects.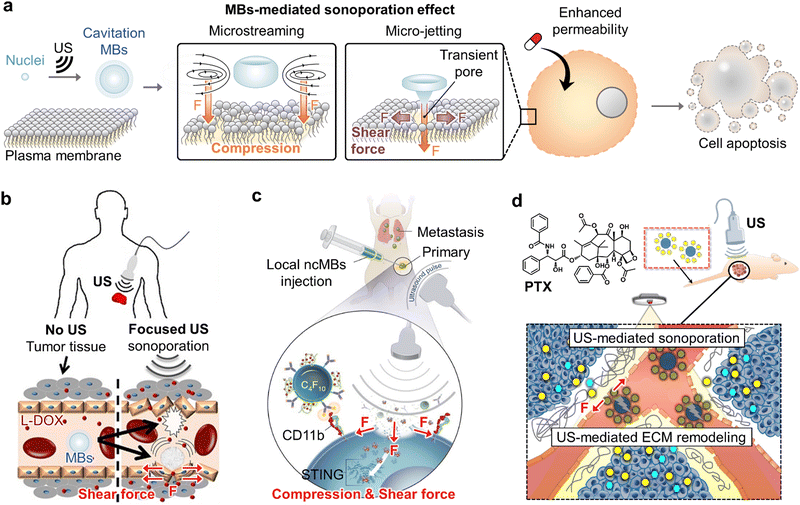 | ||
| Fig. 2 Sono-compression and shear force-mediated cancer therapy. (a) Schematic illustration of sono-compression and shear force-mediated sonoporation to enhance anti-cancer therapeutic strategies under ultrasound irradiation. (b) Graphical illustration of combining sonoporation to enhance the chemotherapeutic effect with liposomal doxorubicin (L-DOX). Reproduced with permission from ref. 30. Copyright, 2020 Ivyspring International Publisher. (c) Schematic representation of nano-complex-decorated MBs targeting CD11b on antigen-presenting cells (APCs) under ultrasound exposure to activate synthase-stimulator of interferon genes (STING) and downstream antitumor immunity. Reproduced with permission from ref. 31. Copyright, 2022, Nature publishing group. (d) Schematic illustration of ultrasound-responsive nanocomplex composed of siRNA-NP and PTX-MBs under the sonopermeable effect for enhanced gene and chemotherapy. Reproduced with permission from ref. 32. Copyright, 2020, Elsevier. | ||
In 2020, Sirsi's group reported MB-assisted ultrasound imaging for monitoring the effects of sonoporation and improving the efficacy of liposomal drug uptake in the context of neuroblastoma.30 Currently, most cancer-targeting nanomedicines focus on passive accumulation as an enhanced permeability and retention (EPR) effect. To overcome the reliance on the EPR effect, a combination of ultrasound and therapeutics has been utilized. This strategy was first introduced based on liposomal doxorubicin (L-DOX, or DoxilTM) which is an FDA-approved nanomedicine and results in an effective delivery using FU and MBs by compression- and shear force-mediated sonoporation without thermal damaging of the tumor vascular network that is essential for continuous chemotherapy delivery to tumors beyond the brain (Fig. 2b).30
Lux et al. developed nanocomplex-conjugated antigen-presenting cell (APC)-targeting MBs for new immunotherapeutic targeting regulators of the innate immune system in cancer. Ultrasound-mediated sonoporation demonstrated a targeted activation synthase-stimulator of interferon genes (STING) platform as a robust strategy for MB-assisted ultrasound-guided immunotherapy of cancer (MUSIC).31 The STING pathway is essential for innate and adaptive immune responses through APCs in cancer. To date, STING antagonists have raised concerns regarding the limit of entry into the cytoplasm and systemic toxicity. However, they proposed a combination of MB-mediated ultrasound and a method to overcome these limitations. The MUSIC platform was the first image-guided cancer immunotherapy that enhanced STING activation following compression- and shear force-induced sonoporation in APCs via drug delivery using specific antibody targets (Fig. 2c). Image-guided immunotherapy using ultrasound-responsive biomaterials generates robust antitumor effects while minimizing systemic toxicity through efficient and targeted potent immune activation for application in various cancers.
Among nanomedicine combination therapies, chemo-gene therapy is emerging as an approach for enhanced anti-cancer efficacy due to its synergistic effects and reduced chemotherapy doses. Nevertheless, effective co-delivery of drugs and therapeutic genes and drugs into targeted cells and tissues is still a limitation. The extracellular matrix (ECM), one of the components of the tumor microenvironment, also inhibits the effects of chemo-gene therapeutics by preventing chemical gene therapy through the infiltration of blood vessels into the cell layers of the tumor. To overcome the limitations of chemo-gene combination therapy, Kim's group has applied an FU-responsive nanocomplex that is composed of siRNA-nanoparticles and paclitaxel-loaded MBs (PTX-MBs) for enhanced chemo-gene therapeutics.32 FU plays an essential role as a non-invasive, safe, and potential therapeutic method. MBs induce the sonoporation effect by FU exposure and loosen the ECM structure via a strong mechanical effect with the nanocomplex for improved penetration of chemo-gene therapeutics (Fig. 2d). As a result of the enhanced FU-mediated dual delivery system, a dramatic antitumor effect was demonstrated by the sonoporation. These strategies are also effective for use in other cancers and are therefore potential therapeutics for controlling dosing and predicting outcomes in clinical applications.
2.3 Sono-tension-mediated cancer therapy
The principle of HIFU treatment is the use of a focused transducer to generate ultrasound and sound waves through the constant temperature of deoxygenated water transmission to the body and convergence at the lesion to form a high-energy acoustic field. In the process of ultrasound propagation, the ultrasound and tissue delivered to the human body converge at the lesion to form a high-energy acoustic field within the tissue. The interaction between ultrasound and tissue can cause effects such as the vibration of the mass of the medium, the absorption of sound energy by biological tissue, and ultrasound “heating” generated by the bubbles.33 The temperature of the tumor is instantaneously increased to the intensity of protein denaturation of the target tissue. This ultimately achieves the purpose of tumor ablation. Thus, two main treatment mechanisms include thermal and mechanical effects of the mechanical force of bubble cavitation. In particular, the mechanical effect is the most primitive effect of any frequency acoustic wave caused by the forward propagation of ultrasound in tissues under reflected waves. Ultrasound vibration causes the tissue to alternate back and forth between compression and extension, and its relative motion causes friction between the masses. The vibration of the masses leads to volume changes and frictional heat generation within the cells.33 The mechanical effect of HIFU is induced by negative pressure that causes MBs to form in the cytoplasm under FU irradiation to achieve pulsatile swelling (tension). Additionally, MBs increase in size to the point at which resonance is achieved, and high pressures of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–30
000–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 bars develop when these MBs accidentally collapse, thus inducing “tension” force-based damage to nearby cellular structures (Fig. 3a). For example, various materials display specific drug release and enhanced tumor uptake, thus providing high drug availability within the tumor microenvironment to cooperate with HIFU ablation.
000 bars develop when these MBs accidentally collapse, thus inducing “tension” force-based damage to nearby cellular structures (Fig. 3a). For example, various materials display specific drug release and enhanced tumor uptake, thus providing high drug availability within the tumor microenvironment to cooperate with HIFU ablation.
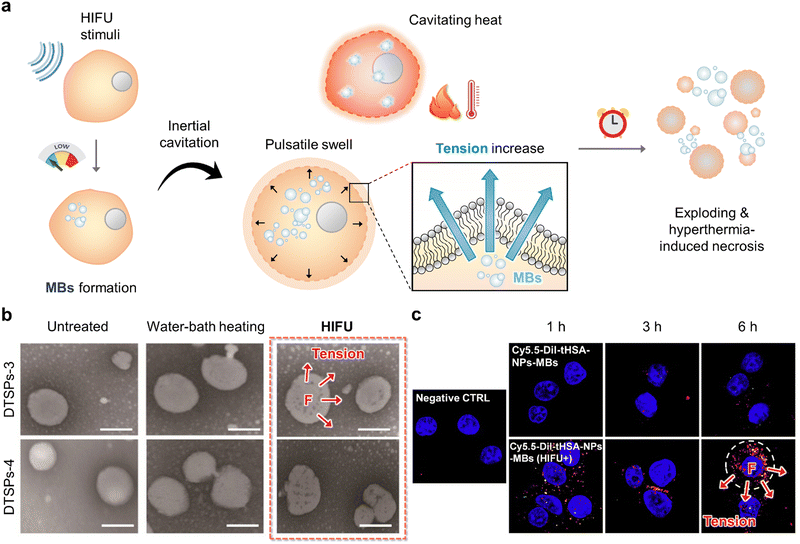 | ||
| Fig. 3 Sono-tension-mediated cancer therapy. (a) Schematic illustration of tension and hyperthermia cytotoxic effect by utilizing high-intensity focused ultrasound (HIFU) irradiation. (b) Graphical representation and transmission electron microscopy (TEM) images of DTSP-3 and DTSP-4 after non-treatment, water bath heating (42 °C, 2 min), and HIFU-irradiation that indicate the HIFU-based cellular tension and hyperthermia damage to cancer cells. The scale bar represents 100 nm. Reproduced with permission from ref. 34. Copyright, 2019, Ivyspring International Publisher. (c) Graphical representation of enhanced cellular uptake of Cy5.5-DiI-tHSA-NPs-MBs and tension-mediated damage with either ultrasound or HIFU exposure. Green and red fluorescence signals indicate DiI and Cy5.5, respectively. Reproduced with permission from the ref. 36. Copyright, 2017, Elsevier. | ||
Jing et al. constructed nano-vesicular temperature-sensitive platelesomes that mimic liposomes by integrating 1,2-dipalmitoyl-sn-glycero-3-phosphocholine, 1-stearoyl-2-hydroxy-sn-glycero-3-phosphocholine, and platelets membrane.34 TSPs can provide higher tumor drug utilization during HIFU thermotherapy in combination with HIFU ablation to provide effective chemotherapy. Most importantly, after HIFU ablation, TSP can spontaneously localize to the postoperative tumor site by adhering to the damaged tumor vessels, thus resulting in ideal consolidation chemotherapy (Fig. 3b). Furthermore, Zheng's group designed a multifunctional therapeutic nanoplatform with HMPBs encapsulating DOX and PFH to achieve in vivo synergistic effects of HIFU/chemotherapy.35 When stimulated by HIFU, PFH bubbles released through mesoporous channels in the HMPB shell can alter the acoustic environment and induce cavitation effects, thereby improving the performance of ultrasound imaging and HIFU therapy.
Han et al. demonstrated HIFU-mediated mechanical stimulation and DDS that was composed of PTX-tHSA-NPs and MBs to achieve enhanced therapeutic efficacy.36 HIFU exposure exhibited superior chemotherapeutic efficacy due to the greater cavitation effect of HIFU in conjunction with MBs. Additionally, HIFU may induce significant thermal- and tension-mediated mechanical damage under the HIFU stimuli (Fig. 3c). Li's group developed a multifunctional nanoparticle-based Fe3O4-PFH/PLGA for HI FU synergistic tumor surgery.37 Due to the integration of superparamagnetic Fe3O4 nanoparticles within the shell and the phase change properties of the PFH core, these nanocapsules were further developed as contrast agents for ultrasound, MR, and PA trimodal imaging and were also used as synergistic agents for enhanced HIFU ablation. In contrast, the combination of transarterial chemoembolization and nanoparticle-enhanced HIFU surgery can effectively improve the treatment outcome of liver cancer, thus providing a new cancer treatment option.
2.4 Sono-shear force-mediated cancer therapy
The energy is emitted in the form of sonoluminescence due to the implosion of bubbles by insonation. It can excite the sonosensitizer to triplet state via intersystem crossing, or from the balance band to the conduction band to achieve energy/electron transfer to tissue oxygen.28 Generated ROS-mediated oxidative stress leads to LPO that is initiated by the attack on a long-chain polyunsaturated fatty acid or fatty acyl side chain of any chemical species that possesses sufficient reactivity with ROS.28,38 Following their reaction with ROS, lipids can combine if they meet, or they can attack membrane proteins. LPO interrupts the interaction between lipoid chains, and this induces mechanically unaligned forces pushing one part of a body in one direction and another part of the body in the opposite direction similar to “induced-shear force” (Fig. 4a). Hence, structural collapse occurs on the cellular membrane to generate cell damage via ferroptosis.39 Ferroptosis is defined as non-apoptotic cell death and is gradually emerging as an important anti-cancer treatment.40 It is induced by the build-up of ROS and LPO products to deadly levels, thus producing an oxidation–reduction imbalance in cells and amplifying ferroptosis effects. Several recent studies have demonstrated the versatile therapeutic potential of nanomaterials that reveal induced-shear force as a sonodynamic effect causing LPO and ferroptosis.41–44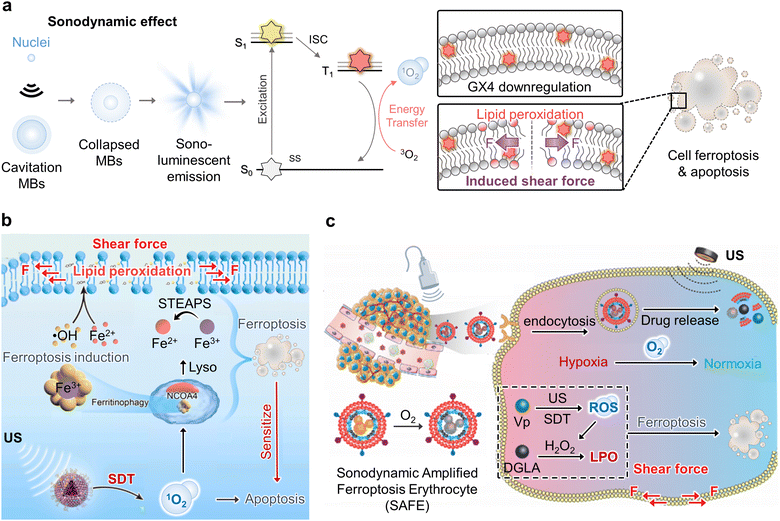 | ||
| Fig. 4 Sono-shear force-mediated cancer therapy. (a) Schematic illustration of ultrasound stimuli-induced lipid peroxidation (LPO) of long-chain polyunsaturated lipid structure as an induced shear force on the plasma membrane for cancer therapy. (b) Graphical representation of the synergistic therapeutic mechanism of sonodynamic therapy (SDT)-based ferroptosis targeting. The targeted delivery of iron oxide nanoparticles ferumoxytol exhibits a ferroptosis-inducing function by importing iron ions and SDT for LPO-induced membrane shear force, thus promoting cellular-selective autophagy. Reproduced with permission from ref. 45. Copyright, 2021, Elsevier. (c) Schematic illustration of induced shear force by utilizing sonodynamic amplified ferroptosis erythrocyte (SAFE) that was composed of an iRGD peptide and red blood cell membrane (RBCM) hybrid camouflaged sono-responsive therapeutic nanocomplex of hemoglobin (Hb), perfluorocarbon (PFC), ferroptosis activator (dihomo-γ-linolenic acid, DGLA), and sonosensitizer (verteporfin, Vp) for the combination treatment of SDT and ferroptosis under ultrasound stimulation. Reproduced with permission from ref. 46. Copyright, 2022, John Wiley & Sons. | ||
In 2021, Chen et al. demonstrated synergistic tumor treatment via an SDT-based LPO mechanism to induce ferroptosis using ferumoxytol and a PpIX sonosensitizer (Fig. 4b).45 As a result, surged unstable Fe2+ in tumor cells caused cell death mediated by ROS-induced ferroptosis. Unlike other typical mechanisms of cell death, such as apoptosis, necrosis, and autophagy, ferroptosis causes substantial damage to mitochondrial shapes such as volume shrinkage and increased membrane density. Furthermore, endogenous biological metabolites have been identified as potential ferroptosis-inducing agents that produce intracellular lipid peroxides, thereby increasing cell vulnerability to ferroptosis. Dihomo-linolenic acid (DGLA) is generated in vivo from the essential fatty acid linolenic acid and functions as a ferroptosis substrate that can be oxidized by ROS in irreversible intracellular LPO.
Regarding the mechanism of shear-force-driven deformation of lipid membranes, vesicles adapt to the applied shear force by creating stress in the membrane and deforming it into an elliptical shape. The shear force-stressed cells appeared to exhibit orientation and extension along the flow direction. Zhou et al. reported sonodynamic-amplified ferroptosis erythrocytes (SAFEs) that exhibited sonodynamic peroxidation of lipids for improving ferroptosis therapy (Fig. 4c).46 In particular, ultrasound-targeted MB is an effective method that combines low-intensity ultrasound with MBs (UTMD). UTMD medication delivery is a targeted technique compared to standard systemic chemotherapeutic drug administration. When external ultrasound irradiation is focused on a tumor, only MBs passing through the beam interact with the ultrasound energy, thus allowing targeted medicine delivery.
In 2022, Zheng and co-workers reported an ultrasound-mediated catalytic and chemotherapeutic liposomal nanomedicine by encapsulating an effective iron-based Fenton catalyst GA-Fe(II) and DOX for synergistically augmenting DOX-resistant breast cancer cells (MCF-7/ADR).47 It exhibited a significant therapeutic effect via a complementary ferroptosis/apoptosis induction modality. GA–Fe(II) complexes can constantly catalyze ˙OH production that is attributed to the depletion of GSH, accumulation of intracellular iron, and enhanced generation of induced-shear force by LPO to actively trigger the iron-dependent ferroptosis pathway within tumor cells. Similarly, Gan et al. demonstrated a versatile manganese porphyrin-based metal–organic framework (Mn-MOF) nanoplatform for enhanced SDT and LPO-based ferroptosis.48 Mn-MOF exhibited high catalytic activity that catalyzes tumor overexpressed H2O2 to generate O2 for tumor hypoxia relief. Meanwhile, Mn-MOF decreased intracellular GSH concentration and GPX4 activity, and this led to enhanced SDT and LPO to achieve significant induced-shear force and cancer cell ferroptosis.
3. Magnetic field
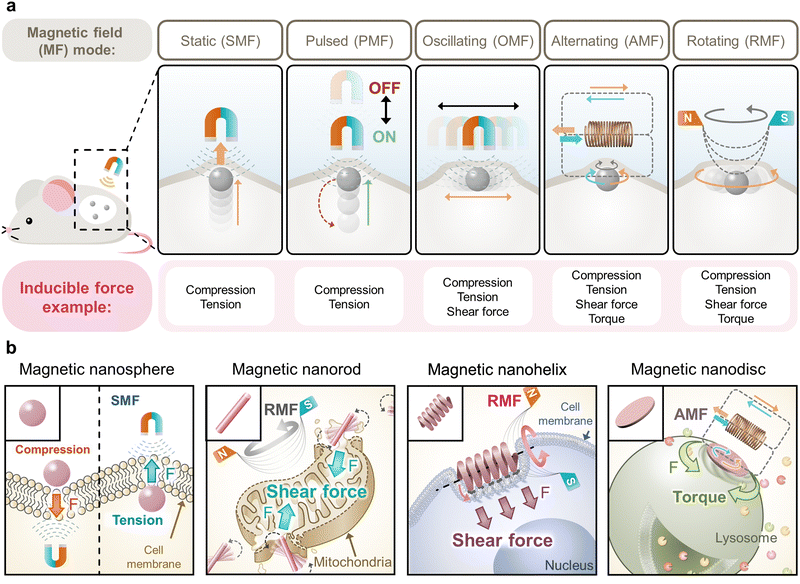
The application of an external magnetic field can exert a magnetic force on magnetic materials that modulates the magnetic spin alignment within the materials, thereby manipulating the motion of magnetic materials.49,50 This magnetically controlled motion of magnetic materials can exert mechanical forces on cancer cell membranes and organelles to trigger mechanically induced cancer cell apoptosis.51 This session will discuss recent developments in such “magnetic field-based mechanical cancer therapy” that have been diversified with the tuning of shapes of magnetic nanomaterials (MNMs) and magnetic field modes.The magnetic field has been utilized to modulate the movement of MNMs in various shapes (e.g., nanospheres, nanorods, nanohelices, nanodiscs, etc.) for cancer therapeutics, tissue regeneration, immunoregulation, and diagnostic biomedical applications52–57 due to their high tissue-penetrative, biocompatible, and spatiotemporally controllable characteristics.58,59 In most cases, MNMs are chemically functionalized with drugs and/or ligands to precisely deliver therapeutic agents, modulate cellular responses, or increase imaging contrast for enhanced efficiency. In general, various mechanical motions of the MNMs can be controlled by changing the applied magnetic field modes, where a static magnetic field (SMF) induces directional movement, a pulsed magnetic field (PMF) induces discrete directional movement, an oscillating magnetic field (OMF) induces periodic movement (oscillation), an alternating magnetic field (AMF) induces spin and vibration, and a rotating magnetic field (RMF) induces rotation about a point and vibration (Fig. 5a).60–64 The AMF flips the magnetic field direction while the RMF rotates the magnetic field direction. Hence, magnetic field modes can be selected for the required mechanical motion to enable precise cancer therapy.65
3.1 Tuning of MNM shape and magnetic field modes for versatile mechanical cancer therapy
The mechanical motion of the MNMs (e.g., movement, oscillation, vibration, spin, and rotation about a point) can be diversified by tuning the shape and functionalization of the MNMs that exert diverse mechanical forces (e.g., compression, tension, shear force, and torque) on cancer cell membranes and organelles for versatile mechanical cancer therapy (Fig. 5b).66 The external magnetic field dynamically modulates the alignment of magnetic spin and dipole moment in the MNMs. In particular, this response is reversible in superparamagnetic MNMs that are observed in small MNMs composed of a single magnetic domain, and this is desirable for controlling the reversible magnetic motion of MNMs in mechanical cancer therapy.In general, MNM shape-dependent motion is tunable with highly anisotropic MNMs exhibiting the shape of the nanorods, nanohelices, nanodiscs, and others that have been actively programmed for microrobot applications.49,50,67,68 They exhibit preferential magnetization along the length axis over the diameter axis in the cases of “nanorod” and “nanohelix” shapes and along the basal plane over out of plane in the case of “nanodisc” shape. Therefore, selectively controlling both the shape of the MNMs and the applied magnetic fields can modulate the dominant types of mechanical forces exerted on cells. Compression and tension can be vertically exerted on cell membranes or organelles by the MNMs in any shape, including magnetic nanospheres under the SMF and PMF, while the MNMs in sharper shape can apply higher compression on cells that are more cell-penetrative.60,61,69 The rubbing effect (shear force and compression) can be laterally applied to cell membranes or organelles by the MNMs under the OMF, AMF, and RMF.70 The spin and rotation about a point of the MNMs arise from the rotation of their magnetic spin and moment (μ) to directionally align themselves with the changing external magnetic field (B).65 Typically, the AMF induces their spinning that possibly applies torque to cells, and the RMF induces their rotation about a point that possibly applies a shear force to cells. Shear force and localized compression can be exerted to “slice” cells by the propeller-like motion of “magnetic nanorods” due to preferential rotation of magnetic spins that are easily aligned along the length axis under the AMF.55,71 Shear forces can be spirally applied to “mill” cells by the spinning of the “magnetic nanohelices” around their major axis due to preferential rotation and translation of magnetic spins that are easily aligned along the curved direction of nanohelices under the RMF.72,73 Shear force and extensive compression have been applied to “scrap” cells by the rotation of the “magnetic nanodiscs” possessing flat morphology due to preferential rotation of magnetic spins that are readily aligned along the basal plane under the AMF.54
Furthermore, the shape-specific mechanical effect exerted on cells can be synergized with the chemical functionalization of MNMs to target or bind to specific cell membranes or organelles. When the MNMs are bound and confined to the targeted organelles, the motion of the MNMs can directly exert mechanical forces on cells such as inflicting localized compression and tension (vibration) under the OMF, localized torque under the AMF, or localized shear force under the RMF.66,74 Additionally, depending on the magnitude of the imposed forces and targeted organelles, such forces can damage cellular structures or stimulate mechanosensitive receptors and ion channels for therapeutic purposes.75 Specifically, forces exerted on cell organelles temporarily deform them until the forces surpass their tolerance to disrupt them. Typically, a pN scale of force is required for the modulation of cells such as several tens to hundreds of pN to rupture cell membranes, 500 pN to disrupt lysosomal membranes, 320 pN of torque or 600 pN of tension to break the actin–actin bonds, and several pN to activate mechanosensitive ion channels.61,64,66,74 Ultimately, these forces result in cell apoptosis, necrosis, or activation to induce cancer cell apoptosis, necrosis, or immune cell activation for cancer immunotherapy. Therefore, the specific desired mechanical motion of the MNMs can be precisely tailored with versatile tuning of their shape, functionalization, and applied magnetic field modes, and they can offer novel modalities for magnetic field-mediated mechanical cancer therapy.
3.2 Magnetic compression- and tension-mediated cancer therapy
Compression and tension are pressing and pulling forces, respectively, that can physically act on cell membranes or organelles. Such forces can be exerted on cells through various mechanical motions of the materials such as directional movement of the MNMs that press or pull cellular structures depending on their targeted state and vibration of the MNMs that in turn vibrates the cell organelles to which they are bound. Typically, compression results in cellular membrane perturbation that damages cells, while tension and vibration (compression and tension) activate ion channels or signaling pathways to regulate cellular responses (Fig. 6a).59,76 Careful control of the mechanical motion of MNMs by altering their shape and functionalization and applied magnetic field modes can modulate the magnitude of the mechanical force exerted on the cells, and this can affect the regulation of cellular function from activation to destruction for effective cancer therapy.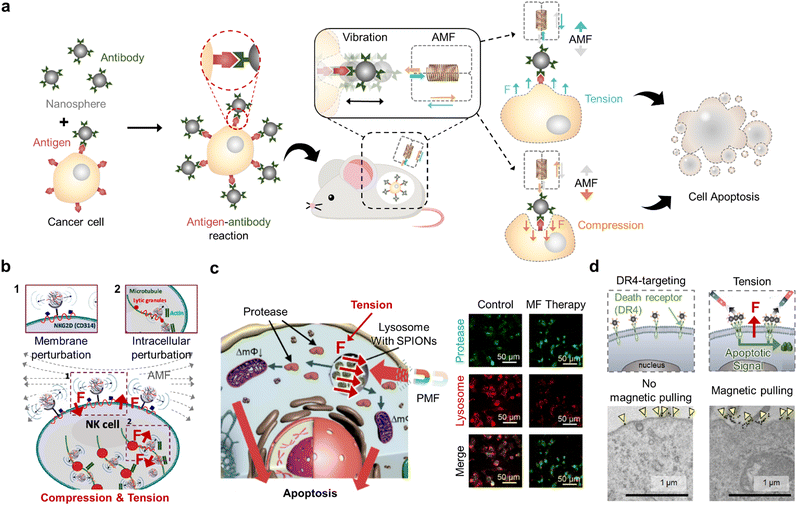 | ||
| Fig. 6 Magnetic compression- and tension-mediated cancer therapy. (a) Schematic illustration of magnetic nanosphere-induced cancer cell apoptosis through the compression and tension exerted on the cell membrane under an alternating magnetic field (AMF). (b) Graphical illustration of NK cell activation through vibration (compression and tension) imposed on the cell membrane under the AMF. Reproduced with permission from ref. 77. Copyright, 2021, American Chemical Society. (c) Schematic representation and immunofluorescence images of cancer cell apoptosis through lysosome permeabilization using magnetic nanosphere-induced tension on the lysosomes of cancer cells under the pulsed magnetic field (PMF). Scale bars: 50 μm. Reproduced with permission from ref. 61. Copyright, 2019, MDPI. (d) Schematic illustration and TEM images of tension acting on the cancer cell membrane by pulling the magnetic nanosphere-bound receptors using a static magnetic field to induce apoptotic signals in cancer cells. Scale bars: 1 μm. Reproduced with permission from ref. 78. Copyright, 2016, American Chemical Society. | ||
For example, it was recently reported that sharp magnetic materials can induce cancer cell necrosis in its bare state or apoptosis in its functionalized form under RMF.69 As the materials were adsorbed on the cell membrane and internalized, their vibration via RMF (2 Hz, 600 mT) imposed localized compression and tension on the cell membrane to decrease cell membrane integrity, ultimately resulting in lactate dehydrogenase leakage as high as 96%. When the reported material was functionalized with poly(ethylene glycol) (PEG), the PEG chains provided steric hindrance to mitigate direct cell-damaging effects caused by the sharp edges. Bossis's group reported the necrosis of cancer cells via compression in vibration when treated with magnetic nanospheres under OMF (10 Hz, 200 mT).76 In detail, liver carcinoma cells were supplemented with a cell culture medium containing the spindle-like particles (1% mass ratio) and subjected to the vertically oscillating magnetic field. Simple indentations made by particle compression are not sufficient to cause membrane damage. However, the aggregation of the particles observed upon oscillating magnetic field exposure generated pressure caused by particle clustering that physically damaged the cell membrane to induce necrosis. These results confirm that the structure of MNMs can alter the cell-damaging mechanism and must be carefully selected to precisely modulate their therapeutic effect.
In 2021, specifically targeted mechanical vibration was introduced for cancer immunotherapy (Fig. 6b).77 Magnetic nanocomplexes were composed of hyaluronic acid and protamine to allow NK cell labeling and ferumoxytol to endow magnetic controllability. The application of AMF (1 Hz, 1.5 mT) vibrated the nanocomplexes in compression and tension, which stimulated mechanosensitive structures of NK cells such as actin and natural killer group receptors, thereby upregulating their cytolytic activity. Practical in vivo application of magnetic NK cell activation in a rat tumor model confirmed its therapeutic efficiency by suppressing the tumor growth rate, thus suggesting the potential of exerting vibration (compression and tension) for cancer immunotherapy. Tension was also introduced on the lysosomes of cancer cells for their disruption.61 In this research, magnetic nanospheres were loaded into the lysosomes of cancer cells through endocytosis and subjected to the PMF of high strength (8 T) and short pulse width (15 μs) (Fig. 6c). The nanospheres were pulled towards the external magnetic field in a pulsed manner, thereby imposing pulsed tension on the lysosomal membrane. Ultimately, the cell membrane was permeabilized, and cathepsin B (protease) leaked into the cytoplasm to induce cancer cell apoptosis. Cheon's group applied tension to facilitate cell apoptosis by magnetically activating death receptor 4 on cancer cells (Fig. 6d).59 The magnetic nanospheres bind to the receptor via an antibody reaction, and the SMF (200 mT) was applied to pull (applying tension) and cluster the magnetic nanosphere-bound receptors without disrupting other cytoskeletal structures. Notably, apoptosis signaling was proportional to SMF strength, with increasing strength causing more receptors to be pulled together in vivo. Subsequently, a death-inducing signaling complex was formed and caspase-3 was activated, ultimately resulting in cancer apoptosis. This approach was also effective in treating multidrug-resistant cancers, thus supporting the clinical applicability of applying tension in cancer therapy.78
3.3 Magnetic shear force-mediated cancer therapy
Shear force is a force involved in “cutting” motion that causes one part of a given cell organelle to be pushed in the opposite direction to its other part. Among various motions, the spin and rotation about a point of the MNMs (primarily caused by the RMF) can representatively impose a shear force by rubbing, slicing, scraping, or milling cell organelles depending upon the MNM shapes and the applied magnetic field.55,70,72,79 In general, such motions can cause cell apoptosis or necrosis by tearing and destroying cellular structures that directly induce leakage of cellular components (Fig. 7a). However, the extent and mechanism of mechanical shear can differ with different combinations of MNM structures, exposed magnetic field strength, and MNM functionalization, and it is, therefore, critical to customize these parameters to maximize cancer therapeutic efficiency.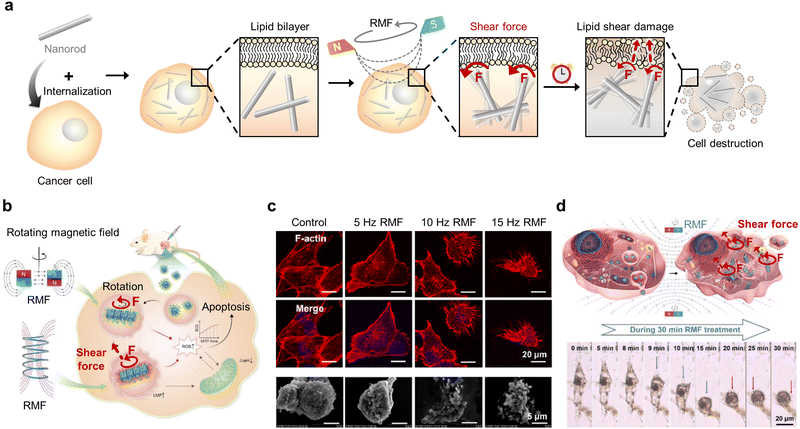 | ||
| Fig. 7 Magnetic shear force-mediated cancer therapy. (a) Schematic illustration of magnetic nanorod-induced cancer cell destruction through shear force exerted on the cell membrane under a rotating magnetic field (RMF). (b–d) Cancer cell apoptosis triggered by shear force generated by the rotation of rod-like magnetic nanocube assembly under RMF exposure. (b) Schematic illustration of shear force-activated ROS production. Reproduced with permission from ref. 82. Copyright, 2020, John Wiley & Sons. (c) Immunofluorescence images and scanning electron microscope (SEM) images illustrating the morphological change of cancer cells after shear force-mediated cancer therapy under RMFs at various frequencies (control, 5, 10, and 15 Hz). The cytoskeleton and nucleus were stained with phalloidin and DAPI, respectively. Scale bars: 20 mm (immunofluorescence) and 5 μm (SEM). Reproduced with permission from ref. 81. Copyright, 2021, American Chemical Society. (d) Schematic illustration and time-dependent images of cancer cell destruction by mitochondrial-targeted RMF treatment. Scale bar: 20 μm. Reproduced with permission from ref. 71. Copyright, 2020, John Wiley & Sons. | ||
In 2021, the rotational motion of rod-shaped MNMs was reported to inflict shear forces on cancer cells to induce apoptosis more efficiently than that induced by spherical MNMs.80 The viability of cancer cells after AMF treatment was significantly lower for those that internalized rod-shaped MNMs compared to that of spherical MNMs. Moreover, the morphological deformation of cancer cells increased when subjected to rod-shaped MNM rotation exerting a shear force, thus demonstrating the MNM shape-dependent effect of mechanical forces exerted on cells. Translation of this rotational shear force in vivo for the treatment of breast tumor-bearing mice resulted in a marked decrease in the relative tumor volume compared to that of the non-treated group. Recently, Cheng's group reported a novel mechanism for cancer cell destruction using magnetic nanocubes that assemble into rod-like morphology and rotate (shear force) upon RMF (15 Hz, 40 mT) exposure (Fig. 7b and c).71,81,82 The nanocubes were designed to be either simply internalized or to target mitochondria for effective cancer therapy. With their interparticle dipolar interactions and geometrical flat surface, exposure to the RMF helped to assemble the magnetic nanocubes into a rod-like morphology. Continuous subjection to the RMF then induces the spinning motion of rod-like nanocube assembly that inflicts a slicing effect (shear force) on cellular organelles.81 The MNMs that do not target specific organelles destroyed the cell membrane and other subcellular structures, thereby activating caspase-3 and elevating ROS production.82 Material functionalization to target mitochondria allowed the localized shear force to be directly imposed upon the mitochondrial membrane, thus resulting in pro-apoptotic proteins such as cytochrome c being leaked into the cytosol (Fig. 7d).71
3.4 Magnetic torque-mediated cancer therapy
Torque is the force exerted on cell organelles when the MNMs are twisted. Typically, the spinning motion of the MNMs that are generally induced by AMF results in the twisting of cellular structures when in direct contact with cellular membranes or when bound to specific organelles (Fig. 8a). Cancer cell death can be induced differently depending on the magnitude of the twisting force. For example, several pN can activate mechanosensitive structures, while hundreds of pN can twist to disrupt cell organelles.74 The degree of twisting can be controlled by altering the MNM morphology, the applied magnetic field frequency, and the targeting ability. Precisely tailoring the torque can diversify the options for effective mechanisms for cancer therapy.83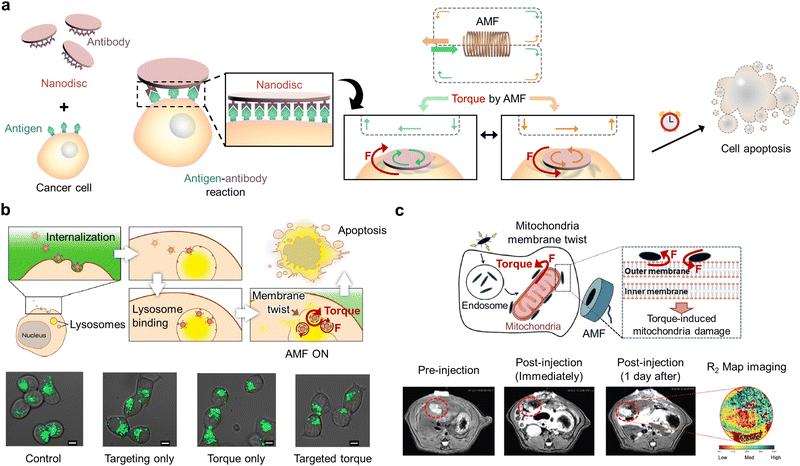 | ||
| Fig. 8 Magnetic torque-mediated cancer therapy. (a) Schematic illustration of magnetic microdisc-induced cancer cell apoptosis by the torque exerted on the cell membrane under an AMF. (b) Graphical representation of the lysosomal membrane rupture through magnetic nanosphere rotation under the AMF to induce cancer cell apoptosis and fluorescence images of lysosomes (LysoTracker Green) in cancer cells in the presence and absence of targeted torque. Scale bars: 5 μm. Reproduced with permission from ref. 84. Copyright, 2014, American Chemical Society. (c) Schematic illustration of the mitochondria damage induced by the torque generated through magnetic nanospindle rotation under the AMF and in vivo T2 magnetic resonance images of pre- and post-injection of MNMs. Reproduced with permission from ref. 85. Copyright, 2021, Royal Society of Chemistry. | ||
In 2022, Carrey et al. reported RMF (1 Hz, 40 mT) treatment of functionalized magnetic nanospheres internalized in cancer cells for tumor therapy.64,74 Specifically, the torque created by nanosphere rotation (3 pN) was not sufficient to directly damage the lysosomal membrane. However, its cumulative exposure was sufficient to disrupt lysosomal integrity, activate ion channels, and induce lysosomal movement. As a result, the targeted cancer cells undergo apoptosis due to lysosomal leakage and microtubule disruption.
To exert torque on cell organelles more efficiently, MNMs were bound to them before they were subjected to AMF. For example, the spinning (torque) of magnetic nanospheres is stimulated when they are bound to lysosomes (membrane twisting) under AMF (20 Hz, 30 mT) (Fig. 8b).63,84 The magnetic nanospheres were functionalized to bind to the lysosomal membrane receptors so that the spinning motion of nanospheres could be directly transmitted to the membrane as twisting energy (torque). As the generated force exceeds membrane tolerance, the membrane tears apart, thus resulting in cancer apoptosis and impaired cancer growth. Master et al. tethered magnetic nanospheres to the lysosomal membranes via polymer network formation to further enhance the effect of torque on cancer cell apoptosis.66 To elaborate, the magnetic nanospheres were coated with polymers to be internalized into cancer cells and anchored to the lysosomal membrane to induce cancer cell cytoskeleton rupture using robust polymer network formation of the MNMs with AMF (50 Hz, 125 mT) for cancer cell destruction. This resulted in the spinning force (torque) of lysosomes being directed to the points of attachment with actin filaments to rupture the cytoskeleton and trigger cancer cell death in response to the external AMF. Different functionalization of MNMs can alter cancer therapeutic mechanisms and effects.
In 2021, Kim's group reported mechanical induction of mitophagy by directly imposing torque on mitochondria (Fig. 8c).85 In detail, magnetic nanospindles were modified with ligands to bind to mitochondria and then subjected to the AMF (0.5–20.0 Hz, 1 mT) that triggered the NMMs to spin (torque) on the mitochondrial surface and damage mitochondrial membranes. Shape-specific mechanical effects contribute to mitochondrial dysfunction. This results in excessive mitophagy that eventually leads to cancer cell death. The spinning of magnetic microdiscs was also introduced to trigger cancer apoptosis by initiating programmed cancer death54 in which the materials were coated with antibodies to attach to the cancer cell membrane and then exposed to AMF (10–20 Hz, 9 mT). Although the twisting force (torque) generated by microdisc spinning was insufficient to rupture the membrane, it triggered apoptosis-related receptors and ion channels. The scrapping effect generated by the spinning of flat and wide disc shapes was considered to activate programmed cell death. Cellular analysis of its morphology and inner ion levels revealed that disturbance of calcium homeostasis via calcium channel perturbation resulted in 90% cancer cell apoptosis.
4. Conclusions and perspectives
We have highlighted the advances in materials and strategies for ultrasound- and magnetic field-driven mechanical cancer therapeutics. From the standpoint of cancer therapeutics, mechanical cancer therapy represents an emerging strategy centered on mechanically stimuli-responsive functional materials and their cytotoxic effects. In recent decades, there has been an exponential increase in mechanical cancer therapeutic applications based on the mechanical stimuli of ultrasound and magnetic field irradiation. It has been reported that the fundamental classification of mechanical forces (compression, tension, shear force, and torque) markedly affects cellular environments to induce apoptotic or non-apoptotic cellular signaling for cancer ablation. The development of fundamental approaches to mechanical cancer therapy will significantly impact cancer treatment and improve overall clinical outcomes with their intrinsic safety.86–88 Additionally, multidisciplinary collaborations, merging photonic cancer therapy, chemical cancer therapy, electric cancer therapy, and other techniques and also gaining access to the most recent developments in mechanical cancer therapy will present a “unique and general” research field.4.1 Outlook for ultrasound-mediated mechanical cancer therapy
Ultrasound-mediated physical interaction with tumor tissue via thermal and non-thermal mechanisms (primarily attributed to acoustic cavitation and implosion) generates various biological effects at the cellular or intact tissue level, ultimately causing structural or functional changes. Ultrasound-mediated mechanical cancer therapy in the cancer region should be considered a practical cancer therapeutic tool due to its deep tumor-penetrative and focusable properties. Indeed, FU- and HIFU-based cancer clinical trials are practically conducted for various cancer patients, including breast, pancreatic, womb, vaginal, cervical, and rectal cancer, within the clinical field of the United Kingdom, and many other versatile approaches were suggested in a large number of research studies.Nevertheless, further progress in the advanced therapeutic applications of ultrasound-mediated cancer therapy requires a better understanding of the physical fundamentals of molecular events and their interactions with tissue-related biological effects. For example, the described cavitation motion of MBs is not well defined. Therefore, it must be precisely characterized to identify possible mechanical forces (e.g., compression, tension, or shear force) responsible for the observed effects. Moreover, it is unknown if ultrasound-driven physical stimulation can substantially exert a significant impact on the cellular structure, and this may provide robust evidence for future ultrasound-mediated mechanical cancer therapy research. Additionally, at lower levels of exposure to ultrasound, there is no established evidence of any specific harmful effects; however, very little research data are available to draw firm conclusions, particularly concerning the long-term use of ultrasound. These subtle effects are not yet entirely understood.
Despite advances in ultrasound, including various physical properties and versatile interaction modes in a biological system, except for imaging techniques this technique has not yet been at the center of clinical applications. Therefore, the development of advanced materials for sonoporation-based DDS, novle sonosensitizers, and techniques of ultrasound machines headed by HIFU make ultrasound-mediated mechanical cancer therapy a promising component in an effort to improve cancer treatment outcomes. Additionally, synergistic combination therapy with conventional anti-cancer therapy provides a promising strategy to compensate for its limitations.
4.2 Outlook for magnetic field-mediated mechanical cancer therapy
The direct application of various MNMs’ motion-mediated mechanical forces to cancer cells via a highly tissue-penetrative magnetic field suggests their clinical cancer therapy applicability with deep tumor-targeting ability. Although numerous recent studies have reported novel and versatile therapeutic modes of magnetic-field-based mechanical cancer therapy, they are in a nascent stage in clinical trials. For example, clinical trials have been initiated in China and the Netherlands to treat patients with osteosarcoma by applying spinning RMF to MNMs to mechanically damage cancer cells. Precise tumor-specific customization of the magnetic field modes and MNMs can offer safe and efficient treatment modalities for magnetic-field-based mechanical cancer therapy.Despite such unprecedented clinical potential, understanding how mechanical forces are exerted on cells is critical for the successful translation of magnetic-field-based cancer therapy. The MNM shape- and functionalization-dependent motion must be further designed and precisely characterized to identify the diverse mechanical forces (e.g., compression, tension, shear force, and torque) imposed on cancer cell membranes and organelles. Calculation of the magnitude of the forces imposed on cells is pivotal for estimating if such forces damage cellular structures or stimulate mechanosensitive receptors and ion channels in cancer cells. Targeting cancer cells must be maximized via MNM functionalization to ensure the safe application of magnetic-field-based cancer therapy.
Magnetic-field-based mechanical cancer therapy can overcome the drawbacks of conventional therapies such as drug tolerance and off-target effects. The versatility of MNMs presents unique opportunities for synergistic combinatorial and imaging-guided therapies. For example, the degradation of MNMs can supply iron ions to trigger the Fenton reaction, thereby enabling chemodynamic therapy in synergy with magnetic field-based mechanical cancer therapy.89 The MNMs can also label cells to enable magnetic resonance imaging-guided mechanical cancer therapy.77 The motion of the MNMs can exert mechanical stimuli to cell membranes to activate ion channels and thus trigger cancer cell apoptosis.54 These combinatorial therapies and imaging-guided therapies coupled with the chemical synthesis of novel MNMs can present unprecedented clinical utilities for magnetic field-based mechanical cancer therapy.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Research Foundation of Korea (No. 2020R1C1C1011038, H. Kang; CRI project No. 2018R1A3B1052702, NRF-2019M3E5D1A01068998, J. S. Kim).References
- L. Yu, Y. Xu, Z. J. Pu, H. Kang, M. Li, J. L. Sessler and J. S. Kim, J. Am. Chem. Soc., 2022, 144, 11326–11337 CrossRef CAS PubMed.
- Y. J. Xu, Y. W. Wang, J. An, A. C. Sedgwick, M. Li, J. L. Xie, W. B. Hu, J. L. Kang, S. Sen, A. Steinbrueck, B. Zhang, L. J. Qiao, S. Wageh, J. F. Arambula, L. P. Liu, H. Zhang, J. L. Sessler and J. S. Kim, Bioact. Mater., 2022, 14, 76–85 CrossRef CAS PubMed.
- P. R. Stauffer, Int. J. Hyperther., 2005, 21, 731–744 CrossRef PubMed.
- T. X. Gu, Y. Wang, Y. H. Lu, L. Cheng, L. Z. Feng, H. Zhang, X. Li, G. R. Han and Z. Liu, Adv. Mater., 2019, 31, 1806803 CrossRef PubMed.
- L. Chen, J. H. Zhang, L. H. Xu, L. C. Zhu, J. P. Jing, Y. S. Feng, Z. Z. Wang, P. F. Liu, W. J. Sun, X. M. Liu, Y. M. Li and H. M. Chen, J. Nanobiotechnol., 2022, 20, 293 CrossRef CAS PubMed.
- N. K. Pandey, W. Xiong, L. Y. Wang, W. Chen, B. Bui, J. Yang, E. Amador, M. L. Chen, C. Xing, A. A. Athavale, Y. W. Hao, W. Feizi and L. Lumata, Bioact. Mater., 2022, 7, 112–125 CrossRef CAS PubMed.
- S. Sengupta and V. K. Balla, J. Adv. Res., 2018, 14, 97–111 CrossRef CAS PubMed.
- S. Durr, C. Janko, S. Lyer, P. Tripal, M. Schwarz, J. Zaloga, R. Tietze and C. Alexiou, Nanotechnol. Rev., 2013, 2, 395–409 Search PubMed.
- Y. Zhang, X. Q. Zhang, H. C. Yang, L. Yu, Y. Xu, A. Sharma, P. Yin, X. Y. Li, J. S. Kim and Y. Sun, Chem. Soc. Rev., 2021, 50, 11227–11248 RSC.
- X. H. Lin, J. B. Song, X. Y. Chen and H. H. Yang, Angew. Chem., Int. Ed., 2020, 59, 14212–14233 CrossRef CAS PubMed.
- M. J. Ko, H. Hong, H. Choi, H. Kang and D. H. Kim, Adv. Nanobiomed. Res., 2022, 2(11), 2200053 CrossRef.
- S. Mukherjee, L. Liang and O. Veiseh, Pharmaceutics, 2020, 12, 147 CrossRef CAS PubMed.
- C. Y. Yang, Y. R. Yu, X. C. Wang, L. R. Shang and Y. J. Zhao, Small, 2022, 18, 2104309 CrossRef CAS PubMed.
- Y. Kim, H. Choi, J. E. Shin, G. Bae, R. Thangam and H. Kang, View-China, 2020, 1, 20200029 CrossRef.
- A. Veith, D. Conway, L. Mei, S. G. Eskin, L. V. McIntire and A. B. Baker, Biomater. Sci., Academic Press, London, Forth edn, 2020, ch. 2.1.6, pp. 717–733 Search PubMed.
- H. Mohammadi and E. Sahai, Nat. Cell Biol., 2018, 20, 766–774 CrossRef CAS PubMed.
- R. K. Jain, J. D. Martin and T. Stylianopoulos, Annu. Rev. Biomed. Eng., 2014, 16, 321–346 CrossRef CAS PubMed.
- B. Coste, J. Mathur, M. Schmidt, T. J. Earley, S. Ranade, M. J. Petrus, A. E. Dubin and A. Patapoutian, Science, 2010, 330, 55–60 CrossRef CAS PubMed.
- Z. Izadifar, P. Babyn and D. Chapman, Ultrasound Med. Biol., 2017, 43, 1085–1104 CrossRef PubMed.
- D. Sharma, K. X. Leong and G. J. Czarnota, Int. J. Mol. Sci., 2022, 23, 4393 CrossRef CAS PubMed.
- S. Ibsen, C. E. Schutt and S. Esener, Drug Des. Dev. Ther., 2013, 7, 375–388 CrossRef CAS PubMed.
- S. M. Chowdhury, L. Abou-Elkacem, T. Lee, J. Dahl and A. M. Lutz, J. Controlled Release, 2020, 326, 75–90 CrossRef CAS PubMed.
- Z. Izadifar, P. Babyn and D. Chapman, J. Med. Biol. Eng., 2019, 39, 259–276 CrossRef.
- R. H. Xiong, R. X. Xu, C. B. Huang, S. De Smedt and K. Braeckmans, Chem. Soc. Rev., 2021, 50, 5746–5776 RSC.
- H. L. Liu, C. H. Fan, C. Y. Ting and C. K. Yeh, Theranostics, 2014, 4, 432–444 CrossRef PubMed.
- Y. Li, Z. Chen and S. Ge, BIO Integration, 2021, 2, 29–36 CrossRef CAS.
- S. Al-Jawadi and S. S. Thakur, Int. J. Pharm., 2020, 585, 119559 CrossRef CAS PubMed.
- S. Son, J. H. Kim, X. W. Wang, C. L. Zhang, S. A. Yoon, J. Shin, A. Sharma, M. H. Lee, L. Cheng, J. S. Wu and J. S. Kim, Chem. Soc. Rev., 2020, 49, 3244–3261 RSC.
- I. Lentacker, I. De Cock, R. Deckers, S. C. De Smedt and C. T. W. Moonen, Adv. Drug Delivery Rev., 2014, 72, 49–64 CrossRef CAS PubMed.
- A. Bellary, A. Villarreal, R. Eslami, Q. J. Undseth, B. Lec, A. M. Defnet, N. Bagrodia, J. J. Kandel, M. A. Borden, S. Shaikh, R. Chopra, T. W. Laetsch, L. J. Delaney, C. M. Shaw, J. R. Eisenbrey, S. L. Hernandez and S. R. Sirsi, Theranostics, 2020, 10, 8143–8161 CrossRef CAS PubMed.
- X. Li, S. Khorsandi, Y. Wang, J. Santelli, K. Huntoon, N. Nguyen, M. Yang, D. Lee, Y. Lu, R. Gao, B. Y. S. Kim, C. de Gracia Lux, R. F. Mattrey, W. Jiang and J. Lux, Nat. Nanotechnol., 2022, 17, 891–899 CrossRef CAS PubMed.
- H. Han, D. Kim, Y. Jang, M. Seo, K. Kim, J. B. Lee and H. Kim, J. Controlled Release, 2020, 322, 346–356 CrossRef CAS PubMed.
- R. J. E. van den Bijgaart, D. C. Eikelenboom, M. Hoogenboom, J. J. Futterer, M. H. den Brok and G. J. Adema, Cancer Immunol. Immun., 2017, 66, 247–258 CrossRef PubMed.
- D. Q. Wu, X. Jin, X. B. Wang, B. Y. Ma, C. M. Lou, H. J. Qu, J. Zheng, B. X. Zhang, X. F. Yan, Y. Wang and L. J. Jing, Theranostics, 2019, 9, 3966–3979 CrossRef CAS PubMed.
- N. Zhang, X. J. Cai, W. Gao, R. H. Wang, C. Y. Xu, Y. Z. Yao, L. Hao, D. L. Sheng, H. R. Chen, Z. G. Wang and Y. Y. Zheng, Theranostics, 2016, 6, 404–417 CrossRef CAS PubMed.
- H. Han, H. Lee, K. Kim and H. Kim, J. Controlled Release, 2017, 266, 75–86 CrossRef CAS PubMed.
- Y. F. You, Z. G. Wang, H. T. Ran, Y. Y. Zheng, D. Wang, J. S. Xu, Z. B. Wang, Y. Chen and P. Li, Nanoscale, 2016, 8, 4324–4339 RSC.
- B. Halliwell and S. Chirico, Am. J. Clin. Nutr., 1993, 57, 715–725 CrossRef PubMed.
- E. Niki, Y. Yoshida, Y. Saito and N. Noguchi, Biochem. Bioph. Res. Co., 2005, 338, 668–676 CrossRef CAS PubMed.
- Y. Xie, W. Hou, X. Song, Y. Yu, J. Huang, X. Sun, R. Kang and D. Tang, Cell Death Differ., 2016, 23, 369–379 CrossRef CAS PubMed.
- C. W. You, X. G. Li, D. Q. Wang, H. Z. Chen, L. Liang, Y. Chen, Y. L. Zhao and H. J. Xiang, Angew. Chem., Int. Ed., 2022, 61, e202210174 CAS.
- Y. D. Lai, N. Lu, A. Ouyang, Q. L. Zhang and P. Y. Zhang, Chem. Sci., 2022, 13, 9921–9926 RSC.
- J. Y. Zhu, A. Ouyang, J. Q. He, J. Xie, S. Banerjee, Q. L. Zhang and P. Y. Zhang, Chem. Commun., 2022, 58, 3314–3317 RSC.
- M. T. Zhu, P. Y. Wu, Y. Li, L. Zhang, Y. J. Zong and M. X. Wan, Biomater. Sci., 2022, 10, 3911–3923 RSC.
- L. Q. Zhou, C. H. Dong, L. Ding, W. Feng, L. D. Yu, X. W. Cui and Y. Chen, Nano Today, 2021, 39, 101212 CrossRef CAS.
- A. Zhou, T. Fang, K. Chen, Y. Xu, Z. Chen and X. Ning, Small, 2022, 18, e2106568 CrossRef PubMed.
- Y. Zheng, X. Li, C. H. Dong, L. Ding, H. Huang, T. H. Zhang, Y. Chen and R. Wu, Adv. Funct. Mater., 2022, 32, 2107529 CrossRef CAS.
- Q. B. Xu, G. T. Zhan, Z. L. Zhang, T. Y. Yong, X. L. Yang and L. Gan, Theranostics, 2021, 11, 1937–1952 CrossRef CAS PubMed.
- S. Palagi and P. Fischer, Nat. Rev. Mater., 2018, 3, 113–124 CrossRef CAS.
- W. Q. Hu, G. Z. Lum, M. Mastrangeli and M. Sitti, Nature, 2018, 554, 81–85 CrossRef CAS PubMed.
- D. Needleman and Z. Dogic, Nat. Rev. Mater., 2017, 2, 1–14 Search PubMed.
- S. Min, M. J. Ko, H. J. Jung, W. Kim, S. B. Han, Y. Kim, G. Bae, S. Lee, R. Thangam, H. Choi, N. Li, J. E. Shin, Y. S. Jeon, H. S. Park, Y. J. Kim, U. K. Sukumar, J. J. Song, S. K. Park, S. H. Yu, Y. C. Kang, K. B. Lee, Q. Wei, D. H. Kim, S. M. Han, R. Paulmurugan, Y. K. Kim and H. Kang, Adv. Mater., 2021, 33, 2008353 CrossRef CAS PubMed.
- Y. Hu, S. Mignani, J. P. Majoral, M. W. Shen and X. Y. Shi, Chem. Soc. Rev., 2018, 47, 1874–1900 RSC.
- D. H. Kim, E. A. Rozhkova, I. V. Ulasov, S. D. Bader, T. Rajh, M. S. Lesniak and V. Novosad, Nat. Mater., 2010, 9, 165–171 CrossRef CAS PubMed.
- A. A. Nana, T. Marimuthu, P. P. D. Kondiah, Y. E. Choonara, L. C. Du Toit and V. Pillay, Cancers, 2019, 11, 1956 CrossRef CAS PubMed.
- S. Y. Liu, X. Chen, L. L. Bao, T. Liu, P. Y. Yuan, X. S. Yang, X. Y. Qiu, J. J. Gooding, Y. K. Bai, J. J. Xiao, F. X. Pu and Y. Jin, Nat. Biomed. Eng., 2020, 4, 1063–1075 CrossRef CAS PubMed.
- A. Manohar, J. Park, D. D. Geleta, C. Krishnamoorthi, R. Thangam, H. Kang and J. Lee, J. Alloys Compd., 2021, 874, 159868 CrossRef CAS.
- Y. Kim, T. M. Koo, R. Thangam, M. S. Kim, W. Y. Jang, N. Kang, S. H. Min, S. Y. Kim, L. T. Yang, H. S. Hong, H. J. Jung, E. K. Koh, K. D. Patel, S. K. Lee, H. E. Fu, Y. S. Jeon, B. C. Park, S. Y. Kim, S. Park, J. Lee, L. Gu, D. H. Kim, T. H. Kim, K. B. Lee, W. K. Jeong, R. Paulmurugan, Y. K. Kim and H. Kang, Adv. Mater., 2022, 34, 110340 Search PubMed.
- M. H. Cho, E. J. Lee, M. Son, J. H. Lee, D. Yoo, J. W. Kim, S. W. Park, J. S. Shin and J. Cheon, Nat. Mater., 2012, 11, 1038–1043 CrossRef CAS PubMed.
- Y. Kim, H. J. Jung, Y. Lee, S. Koo, R. Thangam, W. Y. Jang, S. Y. Kim, S. Park, S. Lee, G. Bae, K. D. Patel, Q. Wei, K. B. Lee, R. Paulmurugan, W. K. Jeong, T. Hyeon, D. Kim and H. Kang, J. Am. Chem. Soc., 2022, 144, 5769–5783 CrossRef CAS PubMed.
- O. Lunov, M. Uzhytchak, B. Smolkova, M. Lunova, M. Jirsa, N. M. Dempsey, A. L. Dias, M. Bonfim, M. Hof, P. Jurkiewicz, Y. Petrenko, S. Kubinova and A. Dejneka, Cancers, 2019, 11, 1873 CrossRef CAS PubMed.
- H. Kang, D. S. H. Wong, X. H. Yan, H. J. Jung, S. Kim, S. Lin, K. C. Wei, G. Li, V. P. Dravid and L. M. Bian, ACS Nano, 2017, 11, 9636–9649 CrossRef CAS PubMed.
- M. Domenech, I. Marrero-Berrios, M. Torres-Lugo and C. Rinaldi, ACS Nano, 2013, 7, 5091–5101 CrossRef CAS PubMed.
- S. Lopez, N. Hallali, Y. Lalatonne, A. Hillion, J. C. Antunes, N. Serhan, P. Clerc, D. Fourmy, L. Motte, J. Carrey and V. Gigoux, Nanoscale Adv., 2022, 4, 421–436 RSC.
- C. Shasha and K. M. Krishnan, Adv. Mater., 2021, 33, 1904131 CrossRef CAS PubMed.
- A. M. Master, P. N. Williams, N. Pothayee, N. Pothayee, R. Zhang, H. M. Vishwasrao, Y. I. Golovin, J. S. Riffle, M. Sokolsky and A. V. Kabanov, Sci. Rep., 2016, 6, 1–13 CrossRef PubMed.
- D. Lisjak and A. Mertelj, Prog. Mater. Sci., 2018, 95, 286–328 CrossRef CAS.
- Y. Kim, H. Yuk, R. K. Zhao, S. A. Chester and X. H. Zhao, Nature, 2018, 558, 274–279 CrossRef CAS PubMed.
- C. Thebault, M. Marmiesse, C. Naud, K. Pernet-Gallay, E. Billiet, H. Joisten, B. Dieny, M. Carriere, Y. Hou and R. Morel, Nanoscale Adv., 2021, 3, 6213–6222 RSC.
- D. Cabrera, A. Coene, J. Leliaert, E. J. Artes-Ibanez, L. Dupre, N. D. Telling and F. J. Teran, ACS Nano, 2018, 12, 2741–2752 CrossRef CAS PubMed.
- M. W. Chen, J. J. Wu, P. Ning, J. J. Wang, Z. Ma, L. Q. Huang, G. R. Plaza, Y. J. Shen, C. Xu, Y. Han, M. S. Lesniak, Z. M. Liu and Y. Cheng, Small, 2020, 16, 1905424 CrossRef CAS PubMed.
- X. H. Yan, Q. Zhou, M. Vincent, Y. Deng, J. F. Yu, J. B. Xu, T. T. Xu, T. Tang, L. M. Bian, Y. X. J. Wang, K. Kostarelos and L. Zhang, Sci. Robot., 2017, 2, eaaq1155 CrossRef PubMed.
- H. W. Huang, F. E. Uslu, P. Katsamba, E. Lauga, M. S. Sakar and B. J. Nelson, Sci. Adv., 2019, 5, eaau1532 CrossRef PubMed.
- A. Hillion, N. Hallali, P. Clerc, S. Lopez, Y. Lalatonne, C. Nous, L. Motte, V. Gigoux and J. Carrey, Nano Lett., 2022, 22, 1986–1991 CrossRef PubMed.
- J. W. Kim, H. K. Jeong, K. M. Southard, Y. W. Jun and J. Cheon, Acc. Chem. Res., 2018, 51, 839–849 CrossRef CAS PubMed.
- B. R. Wang, C. Bienvenu, J. Mendez-Garza, P. Lancon, A. Madeira, P. Vierling, C. Di Giorgio and G. Bossis, J. Magn. Magn. Mater., 2013, 344, 193–201 CrossRef CAS.
- T. Sim, B. Choi, S. W. Kwon, K. S. Kim, H. Choi, A. Ross and D. H. Kim, ACS Nano, 2021, 15, 12780–12793 CrossRef CAS PubMed.
- M. H. Cho, S. Kim, J. H. Lee, T. H. Shin, D. Yoo and J. Cheon, Nano Lett., 2016, 16, 7455–7460 CrossRef CAS PubMed.
- Y. Cheng, M. E. Muroski, D. C. M. C. Petit, R. Mansell, T. Vemulkar, R. A. Morshed, Y. Han, I. V. Balyasnikova, C. M. Horbinski, X. L. Huang, L. J. Zhang, R. P. Cowburn and M. S. Lesniak, J. Controlled Release, 2016, 223, 75–84 CrossRef CAS PubMed.
- N. N. Zhao, L. M. Yan, J. J. Xue, K. Zhang and F. J. Xu, Nano Today, 2021, 38, 101118 CrossRef CAS.
- Y. J. Shen, W. Zhang, G. Li, P. Ning, Z. G. Li, H. T. Chen, X. Y. Wei, X. Pan, Y. Qin, B. He, Z. R. Yu and Y. Cheng, ACS Nano, 2021, 15, 20020–20031 CrossRef CAS PubMed.
- J. J. Wu, P. Ning, R. Gao, Q. S. Feng, Y. J. Shen, Y. F. Zhang, Y. Z. Li, C. Xu, Y. Qin, G. R. Plaza, Q. W. Bai, X. Fan, Z. G. Li, Y. Han, M. S. Lesniak, H. M. Fan and Y. Cheng, Adv. Sci., 2020, 7, 1902933 CrossRef CAS PubMed.
- M. Goiriena-Goikoetxea, D. Munoz, I. Orue, M. L. Fernandez-Gubieda, J. Bokor, A. Muela and A. Garcia-Arribas, Appl. Phys. Rev., 2020, 7, 011306 CAS.
- E. M. Zhang, M. F. Kircher, M. Koch, L. Eliasson, S. N. Goldberg and E. Renstrom, ACS Nano, 2014, 8, 3192–3201 CrossRef CAS PubMed.
- W. Park, S. J. Kim, P. Cheresh, J. Yun, B. Lee, D. W. Kamp and D. H. Kim, Biomater. Sci., 2021, 9, 5497–5507 RSC.
- W. D. O'Brien, Prog. Biophys. Mol. Bio., 2007, 93, 212–255 CrossRef PubMed.
- D. Formica and S. Silvestri, Biomed. Eng. Online, 2004, 3, 11 CrossRef PubMed.
- S. Lee, M. S. Kim, K. D. Patel, H. Choi, R. Thangam, J. Yoon, T. M. Koo, H. J. Jung, S. Min, G. Bae, Y. Kim, S. B. Han, N. Kang, M. Kim, N. Li, H. E. Fu, Y. S. Jeon, J. J. Song, D. H. Kim, S. Park, J. W. Choi, R. Paulmurugan, Y. C. Kang, H. Lee, Q. Wei, V. P. Dravid, K. B. Lee, Y. K. Kim and H. Kang, Small, 2021, 17, 2102892 CrossRef CAS PubMed.
- X. J. Jiang, B. R. Stockwell and M. Conrad, Nat. Rev. Mol. Cell Bio., 2021, 22, 266–282 CrossRef PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |