The spinning processes for spider silk
Xin
Chen
a,
Zhengzhong
Shao
*a and
Fritz
Vollrath
b
aThe Key Laboratory of Molecular Engineering of Polymers, Department of Macromolecular Science, Fudan University, Shanghai, 200433, People's Republic of China. E-mail: zzshao@fudan.edu.cn
bDepartment of Zoology, University of Oxford, South Parks Road, Oxford, OX1 3PS, UK
First published on 9th May 2006
Abstract
This paper summarizes recent work in our groups on the factors that influence the formation of spider silks during the spinning process. The review encompasses: (a) extrusion variables that greatly affect the mechanical properties of the silk filaments; such as rate and temperature at spinning as well as the post-drawn treatment and (b) other factors affecting the conformation transition of the spider silk proteins (spidroin) such as pH and metallic ions. The observations taken together imply that the spinning process is at least as central as, and probably more important than, the composition of the ‘raw’ protein spinning solution. This conclusion leads us to suggest that in the future high-performance, artificial ‘spider’ silks may be spun from a range of solutions of silk and synthetic proteins.
 Xin (Terry) Chen | Xin (Terry) Chen received his BS degree in 1990, MS degree in 1993 and PhD degree in 1996 from Fudan University, China. From 1996 to 2000, he was a lecturer in the Department of Macromolecular Science, Fudan University. In 1999, he was a visiting scientist in Brookhaven National Laboratory, USA. From 2000 to 2001, he was a research assistant in the Department of Zoology, the University of Oxford, UK. Since 2001, he has been an associate professor in the Department of Macromolecular Science, Fudan University. His current research interests include natural polymers and membrane chromatography separation. |
 Zhengzhong Shao | Zhengzhong Shao received his PhD in polymer chemistry and physics from Fudan University, China in 1991. Then he worked as a lecturer in the same university and was promoted to associate professor in 1995. From 1996 to 1998, he was a visiting scientist in the Institute of Biology, Aarhus University, Denmark. Since 1999, he has been a professor in the Department of Macromolecular Science, Fudan University. His current research interests are focused on the animal silks and their proteins, as well as other nature-inspired biomaterials. |
 Fritz Vollrath | Fritz Vollrath obtained his PhD in the Zoology Department at Freiburg University in Germany. After time with the Smithsonian Tropical Research Institution in Panama and the Zoology Departments in Oxford UK, Basel University CH and Aarhus University DK he is now back at the Zoology Department in Oxford where he works primarily on spiders webs and silks. |
1. Introduction
Spiders have spun silk for nearly 400 million years.1 Clearly, silk is very important to the spider because webs, or safety lines spun of silken fibres, accompany all spiders throughout their whole life.2 Among spider silks, dragline (MAjor Ampullate, MAA) silk is most widely studied because of its excellent comprehensive mechanical properties often considered superior to the tough synthetic fibre Kevlar.3 Despite the merits of spider silks such as MAA dragline silk their potential commercial applications are still highly restricted. Practical difficulties with breeding spiders en masse and collecting their silks in commercial quantities are major reasons preventing the deployment of natural spider silks. On the other hand, this provides a great incentive to researchers seeking to create spider silk artificially. In the past 15 years, with the maturation of biotechnological techniques, long gene sequences have been analysed for the principal structural silk proteins of some spider species.4,5 In addition, using recombinant DNA technology, a number of spider silk-like proteins with precisely specified amino acid sequences have been produced.6,7 With those recombinant spider silk proteins (or more simply with ‘regenerated’ spider or silkworm silk proteins) different methods have been explored to spin silk fibres artificially.6,8–10 However, so far most such fibres have much inferior mechanical properties to naturally spun spider silks.3,11 Nevertheless some spinning trials6 managed to produce fibres with comparable modulus and work of fracture albeit with lower tensile strength.In order to artificially mimic spider silk successfully, we need to copy both the spinning dope (the silk protein feedstock) and the spinning process of the spider.3 At first, researchers focused mainly on copying the spinning dope in the belief that (a) the material properties of a silk were a direct consequence of a silk protein's secondary structure and that (b) this structure is determined by the amino acid (gene) sequence.12 However, the poor mechanical properties of the first artificial silks confirm the importance of matching the silk dope (protein sequence and concentration) with the extrusion process.2,6,13 Here, we discuss the evidence for the link between these spinning conditions and the mechanical properties of spider silk.
2. Effect of spinning condition on the mechanical properties of spider silk
Natural spider silks are formed under a mild physiological environment, with an aqueous medium, at ambient temperature and at low hydrostatic pressure on the one hand and with rather slow extrusion rates and low draw-down ratios on the other. The spider's way of spinning and its inability to stop it when artificially ‘silked’ (or reeled) allows us to experimentally test and untangle the various parameters and variables. We place special interest on extrusion rate and spinning temperature because these two factors are key points for the spinning of synthetic, industrial fibres.We studied the two spider species Araneus diadematus and Nephila edulis to analyse the mechanical properties of their MAA dragline silks collected under three different reeling speeds: slow (4.0 mm s−1), medium (20 mm s−1) and fast (100 mm s−1).11 Both breaking strength and initial modulus increased with the increase of reeling speed, while breaking strain decreased. These relations remained when the extrusion speeds were extended even further to range from 0.1 mm s−1 to 400 mm s−1.14 Polymer science can explain this result since increasing reeling speed would lead to ever increasing orientation of the large spidroin molecules.15 Hence we could hypothesize that with increasing extrusion speed the β-sheets would stack more regularly along the long axis of fibre and the molecular chains in the amorphous regions align closer resulting in a stiffer and stronger but less extensible fibre.
But there are other ways to achieve this result. For example, if spiders are induced to spin into water rather than into air, their silk (at the same reeling speed) will be stronger (Fig. 1 (a)).16 This increased strength of underwater spun silks is coupled with increased breaking energy, yield strength and initial modulus but shows decreased breaking strains. Interestingly, loading–unloading tests showed that silks extruded into water exhibit a smaller permanent setting than those spun into air (Fig. 1 (b)) as well as a higher resilience. Again, the changes in mechanical properties between the two treatments must be attributed to the higher orientation of the molecular chains when extruded into water with the assumption that the artificial extension of the spinning duct's aqueous environment gives more time for the spidroin macromolecules to extend and align. This allows for changes of the hydrogen bonds from intra-molecule to inter-molecule manifest in the modified mechanical properties of the fibre. The results showed above support the findings on the relationship between molecule chain orientation and mechanical properties of spider silks in the literatures.17–21
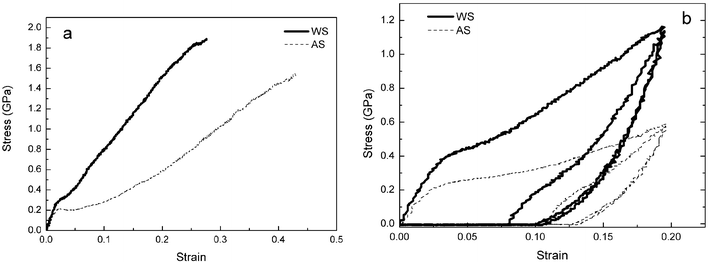 | ||
| Fig. 1 Comparison of the typical properties of spider (Nephila edulis) silk spun at normal speed (20 mm s−1) in air (AS) and in water (WS). (a) stress–strain curves; (b) loading–unloading cycles. (Reproduced by permission of The Royal Society of Chemistry from Chem. Commun., 19, 2489 (ref. 16)). | ||
Temperature at extrusion is another important factor affecting the mechanical properties of the spider's silk. For the ectotherm spider, the highly variable environmental temperature is also the temperature of the fibre formation process and may range from 5–40 °C. Fibres produced over this range show that the energy to break increases with increasing temperature. This is because the breaking strain increases significantly with increasing temperature, although the breaking strength is hardly affected.14 One may speculate that these effects are the result of temperature induced changes in the viscosity of the spinning feedstock, which has been demonstrated in other structural proteins such as ovalbumin.22 Thus, the macromolecular chain of spidroin may have a more relaxed conformation in the amorphous region when the spider silk forms, resulting in the increase of break strain.
3. Other factors involved in natural spinning process
During silk spinning, factors other than the dope composition, spinning speed and temperature are of paramount importance such as pH and metallic ions.23–29 Simply using colored indicators allows a first glimpse at the importance of natural pH changes in the spider's spinning duct indicating a drop from pH 6.9 in the ampulla of the gland to a value of 6.3 in the distal part (third limb) of the duct.23 Employing pH-sensitive microelectrode probes gives a more detailed picture allowing quantitative analysis of the pH gradient in situ at specific positions along the spider's silk secretory pathway.24 Thus it appears that the silk proteins travelling from the gland through the duct (from the tail of the gland to the proximal part of the duct) experience a monotonic decrease in pH from 7.2 to 6.3.24 Hence it may be hypothesised that this acidification of the spinning dope during the extrusion process aids the conformation transition of spidroin from the storage conformation (random coil and/or α-helix) into the final conformation of a mix of α-helix, β-spiral and β-sheet. Rheological studies of the shear behavior of dilute spidroin solutions have confirmed that the conformation transition is achieved at between pH 6.4, and pH 6.8.25 Moreover, circular dichroism (CD) spectra24 confirm that the spidroin in one part of the gland exhibits random coil conformations at a pH = 7.2, whilst it shows β-sheet-rich conformation at the pH 6.3. It appears that the optimal pH value for conformation transition of highly dilute spidroin solution is 4.35 which is remarkably close to the value of isoelectric point (pI, 4.22).26 The differences for the acidic environment optimal for conformation changes around a pH of 4.2 for the concentrated (20%) spidroin solution24 and a pH of 4.4 for the highly dilute (1%) spidroin solution26 probably reflect differences in protein–protein interactions in the different concentration. In summary, all observations and experiment demonstrate that, for silk dope, acidification leads to β-sheet formation.Besides pH, metallic ions are other important factors in the spinning process of the Nephila spider with both Na+ and K+ being present in both the silk duct and fibre.23,27 The K+ content in the silk gland is about 750 µg g−1 and increases along the spinning duct, finally reaching 2900 µg g−1 in the silk fibre while at the same time the Na+ content decreases from 3130 µg g−1 in the gland to 300 µg g−1 in the fibre.27 FTIR, Raman and CD measurements demonstrate that these two metallic ions can separately as well as jointly induce the conformation of spidroin from random coil and/or α-helix into β-sheet.26–30 However, there are differences between the action of Na+ and K+. For example, the addition of K ions induces the formation of spidroin nanofibrils while Na ions seem to have no such effect.25 Moreover, the β-sheet content in membranes cast from extracted spidroin spinning feedstock is found to be higher if induced by K+ as opposed to an induction by Na+.29
4. Conclusion and outlook
Under current spinning technologies, the mechanical properties of artificial spider silk either from regenerated spidroin or from recombinant spidroin solution cannot reach those of natural spider silks. Clearly, in order to successfully spin we have to consider, in addition to the rate and temperature at spinning, also the post-draw ratio and the change of pH as well as the effect of metallic ions, effects that are confirmed by studies using single-fibre Raman spectroscopy31 and micro X-ray diffraction.32,33Moreover, the importance of the ‘right’ spinning conditions is supported by work on the mechanical of silkworm silk.34 Traditionally, commercial silkworm silk is presumed to be much weaker and less extensible than the spider dragline silk. However, fibres artificially reeled from immobilized silkworms under steady and controlled conditions are much superior to fibres spun the natural way,34 (i.e., onto a cocoon wall) and approach some spider silks (Fig. 2). This suggests that, although the amino acid sequence of silkworm silk is rather different to that of spider silk, fibre properties can be similar if spinning conditions are controlled.
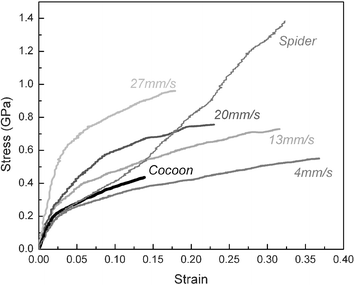 | ||
| Fig. 2 Comparison of stress–strain curves of silkworm silks motor-reeled at the indicated speeds at 25 °C, as well as Nephila spider dragline silk reeled at 20 mm s−1 at 25 °C and standard, degummed commercial silk from a silkworm cocoon. Reproduced by permission of the Nature Publishing group from Nature, 418, 741 (ref. 34)). | ||
The work discussed here suggests that we should be able to produce high-performance artificial silk fibres not only from artificial spidroins, which are (still) some way in the future. Instead it might be possible to produce artificial ‘spider’ silk from the more abundant silk protein produced by commercial silkworms—perhaps using extruders that mimic the natural spinning process.13,35–37
Acknowledgements
For funding we thank the National Natural Science Foundation of China (Nos. 20434010, 20525414 and 50373006), as well as the European Commission (G5RD-CT-2002-00738), the British EPSRC (GR/N01538/01) and the AFSOR of the United States of America (F49620-03-1-0111).References
- C. L. Craig, Biomacromolecules, 2004, 5, 739 CrossRef CAS.
- F. Vollrath, Int. J. Biol. Macromol., 1999, 24, 81 CrossRef CAS.
- F. Vollrath and D. P. Knight, Nature, 2001, 410, 541 CrossRef CAS.
- M. Xu and R. V. Lewis, Proc. Natl. Acad. Sci. U. S. A., 1990, 87, 7120 CAS.
- M. B. Hinman and R. V. Lewis, J. Biol. Chem., 1992, 267, 19320 CAS.
- A. Lazaris, S. Arcidiacono, Y. Huang, J. F. Zhou, F. Duguay, N. Chretien, E. A. Welsh, J. W. Soares and C. N. Karatzas, Science, 2002, 295, 472 CrossRef CAS.
- S. R. Fahnestock and S. L. Irwin, Appl. Microbiol. Biotechnol., 1997, 47, 23 CrossRef CAS.
- J. P. O'Brien, S. R. Fahnestock, Y. Termonia and K. C. H. Gardner, Adv. Mater., 1998, 10, 1185 CrossRef CAS.
- A. Seidel, O. Liivak, S. Calve, J. Adaska, G. D. Ji, Z. T. Yang, D. Grubb, D. B. Zax and L. W. Jelinski, Macromolecules, 2000, 33, 775 CrossRef CAS.
- Z. Z. Shao, F. Vollrath, Y. Yang and H. C. Thogersen, Macromolecules, 2003, 36, 1157 CrossRef CAS.
- B. Madsen, Z. Z. Shao and F. Vollrath, Int. J. Biol. Macromol., 1999, 24, 301–306 CrossRef CAS.
- M. L. Casem, D. Turner and K. Houchin, Int. J. Biol. Macromol., 1999, 24, 103 CrossRef CAS.
- L. Zhou, X. Chen, Z. Z. Shao, Y. F. Huang and D. P. Knight, J. Phys. Chem. B, 2005, 109, 16937 CrossRef CAS.
- F. Vollrath, B. Madsen and Z. Z. Shao, Proc. R. Soc. London, Ser. B, 2001, 268, 2339 CrossRef CAS.
- Y. Liu, Z. Z. Shao and F. Vollrath, Nat. Mater., 2005, 4, 901 CrossRef CAS.
- Y. Liu, Z. Z. Shao and F. Vollrath, Chem. Commun., 2005,(19), 2489 RSC.
- B. L. Thiel, D. D. Kunkel and C. Viney, Biopolymers, 1994, 34, 1085.
- Z. Z. Shao and F. Vollrath, Polymer, 1999, 40, 1799 CrossRef CAS.
- Z. Shao, F. Vollrath, J. Sirichaisit and R. J. Young, Polymer, 1999, 40, 2493 CrossRef CAS.
- J. Perez-Rigueiro, M. Elices and G. V. Guinea, Polymer, 2003, 44, 3733 CrossRef CAS.
- G. V. Guinea, M. Elices, J. Perez-Rigueiro and G. R. Plaza, J. Exp. Biol., 2005, 208, 25 Search PubMed.
- K. Monkos, Biophys. Chem., 2000, 85, 7 CrossRef CAS.
- D. P. Knight and F. Vollrath, Naturwissenschaften, 2001, 88, 179 CrossRef CAS.
- C. Dicko, F. Vollrath and J. M. Kenney, Biomacromolecules, 2004, 5, 704 CrossRef CAS.
- X. Chen, D. P. Knight and F. Vollrath, Biomacromolecules, 2002, 3, 644 CrossRef CAS.
- C. Dicko, J. M. Kenney, D. Knight and F. Vollrath, Biochemistry, 2004, 43, 14080 CrossRef CAS.
- X. Chen, Y. F. Huang, Z. Z. Shao, Y. Huang, P. Zhou, D. P. Knight and F. Vollrath, Chem. J. Chin. Univ., 2004, 25, 1160 Search PubMed.
- X. Chen, D. P. Knight, Z. Z. Shao and F. Vollrath, Biochemistry, 2002, 41, 14944 CrossRef CAS.
- X. Chen, Z. Z. Shao, D. P. Knight and F. Vollrath, Acta Chim. Sin., 2002, 60, 2203 Search PubMed.
- X. N. Peng, Z. Z. Shao, X. Chen, D. P. Knight, P. Y. Wu and F. Vollrath, Biomacromolecules, 2005, 6, 302 CrossRef CAS.
- J. Sirichaisit, R. J. Young and F. Vollrath, Polymer, 2000, 41, 1223 CrossRef CAS.
- C. Riekel, B. Madsen, D. P. Knight and F. Vollrath, Biomacromolecules, 2000, 1, 622 CrossRef CAS.
- C. Riekel and F. Vollrath, Int. J. Biol. Macromol., 2001, 29, 203 CrossRef CAS.
- Z. Z. Shao and F. Vollrath, Nature, 2002, 418, 741 CrossRef CAS.
- A. E. Terry, D. P. Knight, D. Porter and F. Vollrath, Biomacromolecules, 2004, 5, 768 CrossRef CAS.
- L. Zhou, X. Chen, Z. Z. Shao, P. Zhou, D. P. Knight and F. Vollrath, FEBS Lett., 2003, 554, 337 CrossRef CAS.
- X. H. Zong, P. Zhou, Z. Z. Shao, S. M. Chen, X. Chen, B. W. Hu, F. Deng and W. H. Yao, Biochemistry, 2004, 43, 11932 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2006 |
