Effects of cadmium and enhanced UV radiation on the physiology and the concentration of UV-absorbing compounds of the aquatic liverwort Jungermannia exsertifolia subsp. cordifolia
S. Otero, E. Núñez-Olivera, J. Martínez-Abaigar*, R. Tomás, M. Arróniz-Crespo and N. Beaucourt
Universidad de La Rioja, Complejo Científico-Tecnológico, Avda. Madre de Dios 51, 26006, Logroño (La Rioja), Spain. E-mail: javier.martinez@daa.unirioja.es
First published on 20th June 2006
Abstract
The aquatic liverwort Jungermannia exsertifolia subsp. cordifolia was cultivated for 15 d under controlled conditions to study the single and combined effects of cadmium and enhanced ultraviolet (UV) radiation. Both cadmium and UV radiation caused chlorophyll degradation and a decrease in the maximum quantum yield of photosystem II (PSII), together with an increase in the mechanisms of non-photochemical dissipation of energy (increase in the xanthophyll index). Cadmium was more stressing than UV radiation, since the metal also influenced photosynthesis globally and caused a decrease in net photosynthetic rates, in the effective quantum yield of photosynthetic energy conversion of PSII, and in the maximal apparent electron transport rate through PSII. Ultraviolet radiation increased the level of trans-p-coumaroylmalic acid and cadmium increased trans-phaselic and feruloylmalic acids. The increase in these compounds was probably related to both a more efficient absorption of harmful UV radiation and an enhanced protection against oxidative stress. DNA damage was specifically caused by UV-B radiation, but was intensified under the presence of cadmium, probably because the metal impairs the DNA enzymatic repair mechanisms. Ultraviolet radiation and cadmium seemed to operate additively on some physiological processes, while other responses were probably due to either factor alone.
Introduction
Cadmium (Cd) is an important and widespread heavy metal, released into the environment by power stations, heating systems, metal industries, waste incinerators, urban traffic, cement factories, and agricultural fertilizers.1 It occurs also in natural environments as an accompanying metal in Pb and Zn minerals. The general effects of Cd toxicity in higher plants include root alterations, leaf roll, chlorosis, and reduced growth. More specifically, Cd interacts with water balance, damages the photosynthetic apparatus, lowers chlorophyll and carotenoid content, inhibits stomatal opening, affects the activity of several enzymes through replacement of other metal ions, and produces oxidative stress.1 In response to Cd stress, plants can resort to a number of defence systems, such as exudation of complexing agents, immobilization in the cell wall, exclusion through the action of the plasma membrane, compartmentalization in the vacuole, formation of metal-resistant enzymes, and synthesis of phytochelatins and stress proteins.1,2 Cadmium accumulation in plants may be dangerous for animal life and human health because Cd enters the food chains in this way.Ultraviolet (UV) radiation has many harmful effects in all living organisms, including humans. UV-C radiation (<280 nm) is ecologically irrelevant since it is absorbed by atmospheric oxygen and ozone, but both UV-B (280–315 nm) and UV-A (315–400 nm) have significant biological effects, although most UV-B is absorbed by the stratospheric ozone layer. In mid-latitudes, ozone depletion, mainly due to the emission of CFCs, has led to 6–12% increase in UV-B radiation over the 1980 levels, and predicted changes show the ozone layer will remain vulnerable to further depletion in the near future.3 Consequently, studies on the effects of enhanced UV-B levels on photosynthetic organisms are increasingly important. For years, these studies have focused on UV-B, and thus biologically effective UV-B (UV-BBE) was calculated, using appropriate spectral weighting functions to compensate the strongly wavelength-dependent effects of UV radiation.4 However, the current trend is to pay more attention to UV-A wavelengths for calculating biologically effective UV radiation (UVBE), and thus new functions have been developed.5–8 In photosynthetic organisms, UV-B radiation may lead to chlorophyll and carotenoid degradation, PSII damage, photoinhibition, photosynthesis reduction, DNA damage, oxidative stress, and general growth inhibition and development alterations.9,10 However, some controversy still persists regarding the ecological relevance of these effects.11 Photosynthetic organisms can develop several mechanisms against the harmful effects of UV-B, such as mechanical protections (e.g., thick cuticles, multilayered epidermis, hairs), synthesis of UV-absorbing compounds (e.g., phenolic derivatives, mycosporine-like amino acids), antioxidant and photoprotection systems, and DNA repairing mechanisms.10,12 The vast majority of studies in the context of UV radiation have dealt with terrestrial plants, mainly field crops and diverse types of algae,9,13–15 whereas bryophytes have received less attention.
Photosynthetic organisms from both terrestrial and aquatic environments may be exposed to simultaneous Cd and UV stresses, which would cause severe damage since both factors affect basic metabolic processes. However, only a few studies have dealt with the interaction of both factors, and they have been conducted on terrestrial vascular plants,16–19 cyanobacteria,20–22 and a terrestrial bryophyte.23 These studies have documented reductions in chlorophyll content and growth, inhibition of PSII activity, increase in lipid peroxidation, and changes in primary metabolism, mineral contents and antioxidant enzymes.
In the aquatic environment, bryophytes are especially relevant in streams, since they are frequently the most abundant primary producers, support periphyton and provide a refuge, and occasionally food, for macroinvertebrates, amphibian, and fish.24 In addition, bryophytes have been extensively used as bioindicators of heavy metal pollution due to their high accumulation capacity.25 Bryophytes also respond to UV radiation26–32 and could be UV-sensitive because of the lack of structural defences common in vascular plants. Thus, aquatic bryophytes may be notably vulnerable to the combination of heavy metal and UV stresses. However, to our knowledge no previous study has examined this issue, since the only bryophyte studied previously was a terrestrial liverwort.23
The aim of this study was to elucidate the individual and combined effects of Cd and enhanced UV radiation on the physiology of an aquatic liverwort (Jungermannia exsertifolia subsp. cordifolia). The responses to Cd and UV were evaluated in terms of sclerophylly, photosynthetic pigment composition, chlorophyll fluorescence, photosynthesis, respiration, UV-absorbing compounds, and DNA damage. Given that both Cd and UV may induce the synthesis of secondary metabolites with antioxidant and/or UV-absorbing functions, several individual phenolic derivatives previously identified in Jungermannia exsertifolia subsp. cordifolia33 have been studied. This individual analysis may have more physiological relevance than the usual analysis of bulk UV-absorbing compounds,11 because each compound may respond in a different manner. Our hypothesis was that the interaction between Cd and enhanced UV (especially UV-B) would have more harmful effects than each factor separately, and that the plant would synthesize higher amounts of phenolic derivatives to counteract damage. We used enhanced levels of both Cd and UV radiation, with respect to those usually found in nature, to observe clear physiological effects (e.g., DNA damage or accumulation of phenolic compounds) that would otherwise be difficult to detect.
Materials and methods
Experimental design
Unshaded and submerged samples of the foliose aquatic liverwort Jungermannia exsertifolia Steph. subsp. cordifolia (Dumort.) Vána (hereafter J. cordifolia) were collected on 5 April 2005 at the first-order stream Senestillos (1350 m a.s.l., 42°02′ N, 02°37′ W), in La Rioja (northern Spain). The material was rinsed with stream water and transported to the laboratory in a portable icebox (temperature always below 5 °C).Healthy apices were submerged at a depth of 2 cm into 18 perspex tubes with a basal net to prevent material losses. Two groups of 9 tubes were placed in independent recipients filled respectively with air-bubbled stream water (control; pH 6.8, conductivity 21 µS cm−1) and with the same water supplemented with 5 µM Cd2+ (562.0 µg l−1). Cd2+ was measured periodically in both culture media (Perkin-Elmer AA-700 atomic absorption spectrophotometer, Perkin-Elmer, Wilton, CT, USA), which were permanently circulating and kept at 10 °C. Each recipient was placed on a rotating platform to prevent possible place-dependent differences in the radiation received by the plants, which was provided by a Hönle SOL 1200RF2 lamp (Dr Hönle AG UV-Technologie, Gräfelfing/Munich, Germany), Sylvania Coolwhite (Osram-Sylvania, Madrid, Spain), and True-lite full spectrum (True Sun, Steubenville, OH, USA) fluorescent tubes. Spectral characteristics have been published elsewhere.60 Three replicates of the following three radiation regimes for each culture medium were set by covering the tubes with specific UV cut-off foils: (1) P, Photosynthetically active radiation (PAR) alone, using Ultraphan 395 (Digefra GmbH, Munich, Germany), which cuts off all UV radiation, (2) PA (PAR + UV-A), using Folex 320 (Folex GmbH, Dreieich, Germany), which cuts off UV-B and UV-C radiation, and (3) PAB (PAR + UV-A + UV-B), using Ultraphan 295 (Digefra GmbH, Munich, Germany), which cuts off UV-C radiation.
The filters were pre-irradiated for 1 h and replaced every 5 d. Table 1 shows the radiation conditions in the three different regimes. The biologically effective UV-B irradiance (UV-BBE) was estimated using classic and modern action spectra: the generalized plant damage action spectrum4 normalized at 300 nm, the DNA damage spectrum,34 and that of Flint and Caldwell.5 Given that the latter also takes into account UV-A radiation, biologically effective UV irradiance (UVBE) was estimated in this case. The spectral irradiances were measured using a spectroradiometer (Macam SR9910, Macam Photometrics Ltd, Livingstone, Scotland), and PAR was measured with a quantum sensor (LI-190SA). Samples were cultivated with a 12 : 12 photoperiod for 15 d. The UV source (Hönle lamp) was switched on around noon for 7 h per day (square-wave). The plants received a daily UV-B dose of 28.2 kJ m−2, which was required to mimic a 20% ozone depletion as calculated with a computer model.35
Physiological measurements
At the beginning and at the end of the culture period, 9–15 measurements of each physiological variable were taken for each culture medium and radiation regime.The sclerophylly index (SI) was calculated as the quotient between the dry mass (DM: 80 °C for 24 h) and the surface area of the prostrate apex (LI-COR LI-3000 area meter, Lincoln, NE, USA). Previously, fresh mass (FM) was measured.
Spectrophotometric analysis of photosynthetic pigments (Perkin-Elmer λ35 UV/Vis, Perkin-Elmer, Wilton, CT, USA) was performed after extraction with 80% acetone.28 Chlorophyll (Chl a + b) and carotenoid concentrations per unit of DM and shoot area, together with the chlorophyll a/b quotient (Chl a/b), the OD430/OD665 and OD430/OD410 indices (OD is optical density), were obtained. The OD430/OD665 index may be indicative of the photoprotective capacity of bryophytes, increasing in stress situations, whereas the OD430/OD410 index decreases under adverse conditions.36 HPLC analysis of photosynthetic pigments was performed in accordance with De Las Rivas et al.,37 using an Agilent HP1100 HPLC system (Agilent Technologies, Palo Alto, CA, USA) and an Agilent photodiode array detector. For quantification, commercial standards of chlorophylls a and b (Fluka), and lutein, zeaxanthin and β-carotene (CaroteNature) were used. The xanthophyll index (antheraxanthin + zeaxanthin)/(violaxanthin + antheraxanthin + zeaxanthin) and the relationship between (antheraxanthin + zeaxanthin)/Chl a were calculated.
Net photosynthesis (PN) at saturating PAR (500 µmol m−2 s−1) and dark respiration (RD) were measured at 20 °C in an oxygen-electrode (DW2/2, Hansatech Instruments Ltd, King's Lynn, Norfolk, UK), using a 0.1 M NaHCO3 solution as carbon source.28PN and RD rates were calculated per unit of DM and shoot area.
Chlorophyll fluorescence of PSII was measured with a portable pulse amplitude modulation fluorometer (MINI-PAM, Walz, Effeltrich, Germany).38 Minimal and maximal fluorescence (F0 and Fm) were measured before the beginning of the daily light period, and then saturating pulses of a white actinic light (400 µmol m−2 s−1) were applied until Ft and Fm′ stabilized at steady values.28 The maximum quantum yield of PSII was given by the ratio Fv/Fm, where Fv = Fm − F0, and the effective quantum yield of photosynthetic energy conversion of PSII (ΦPSII) by the ratio (Fm′ − Ft)/Fm′. Non-photochemical quenching (NPQ) was determined as (Fm − Fm′)/Fm′. The apparent electron transport rate through PSII (ETR) was calculated as: ETR = ΦPSII × PAR level × 0.5 × 0.84.38 The curves ETR vs. PAR (from 0 to 1600 µmol m−2 s−1) and NPQ vs. PAR were obtained and fitted to Jassby and Platt's equation39 to calculate maximal ETR (ETRmax) and maximal NPQ (NPQmax).
The following individual UV-absorbing compounds of J. cordifolia were extracted and analyzed by HPLC (Agilent HP1100 HPLC system):33 phaselic acid (in cis/trans isomers), trans-p-coumaroylmalic acid, feruloylmalic acid, and two coumarins: 5″-(7″,8″-dihydroxy-7-O-β-glucosyl-coumaroyl)-2-caffeoylmalic acid and 5″-(7″,8″-dihydroxycoumaroyl)-2-caffeoylmalic acid (hereafter, coumarin 1 and coumarin 2, respectively). Concentrations were expressed per unit of DM and surface area. Quercetin dihydrate (Sigma) was added as internal standard.
The concentration of Cd in the liverwort was obtained in triplicate during the culture period (Perkin-Elmer AA-700 atomic absorption spectrophotometer), after digestion of oven-dried tissues in a mixture of HNO3 and H2O2 (Milestone Ethos Touch Control microwave digestor, Milestone Inc., Shelton, CT, USA). To check the accuracy of the analytical procedures, the Certified Reference Material GBW 07604 was used (Institute of Geophysical and Geochemical Exploration, Langfang, China).
DNA damage was evaluated by detection of thymine dimers40 at the end of the light period on the last day of culture. To establish a standard with known thymine dimer frequency, the plasmid pBSK (obtained from Prof. D.-P. Häder, Erlangen, Germany) was used and its DNA was isolated (QIAprep Spin Kit, Qiagen, Hilden, Germany). After quantification, plasmid DNA was irradiated for 1 h with an UV-C irradiance (254 nm) capable of inducing all possible thymine dimers. Once the DNA of both the samples and the irradiated plasmid had been obtained, it was fixed to a Hybond-N+ membrane (Amersham Biosciences, GE Healthcare UK Ltd., Little Chalfont, Buckinghamshire, UK), which was blocked and incubated with the primary antibody (anti-thymine dimer TDM-2, obtained from Prof. Osamu Nikaido, Kanazawa University, Japan). Afterwards, the membrane was incubated with the secondary antibody (antimouse-IgG) using the ECL Western blotting system (Amersham Biosciences). Thymine dimers were detected and quantified by chemiluminiscence using the ChemiGenius Bio Imaging System and associated software (Syngene, Cambridge, UK).
Statistical analysis
The effects of the culture medium and the radiation regime were tested using a 2-way analysis of variance (ANOVA) if the data met the assumptions of normality and homoscedasticity. If not, a Kruskal–Wallis test was used. In the case of significant differences, means were then compared by the Student's t, LSD or Mann–Whitney tests. For each culture medium, 1-way ANOVAs were conducted to analyze the effect of the radiation regime and LSD tests to compare means. For each radiation regime, Student's t tests were applied to analyze the effect of the culture medium. A Principal Components Analysis (PCA) was conducted to rank the final results of the physiological variables obtained for each radiation regime and culture medium, together with the initial data (values were introduced only on a DM basis). All the statistical procedures were performed with SPSS 12.0 for Windows (SPSS Inc., Chicago, IL, USA).Results
In the Cd-treated culture, Cd concentration in the water decreased strongly during the first days of culture and more slowly thereafter, whereas the liverwort tissues accumulated Cd rapidly during the first days and slowly thereafter (Fig. 1). There were no significant differences in the tissue Cd between the three radiation regimes at any moment of the culture period. In the control medium, throughout the culture period, Cd was undetectable in the water (<0.1 µg l−1) and its concentration in the tissues was around 0.10 mg g−1 DM (with no significant difference between the three radiation regimes).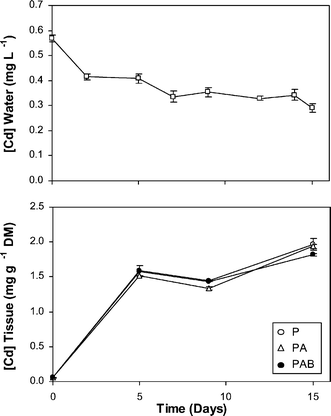 | ||
| Fig. 1 Cadmium concentrations in the water (top) and in the liverwort tissues for the three radiation regimes used, P, PA and PAB (bottom), in the Cd-treated culture along the experimental period. In the control, Cd in water was undetectable (<0.1 µg l−1) and concentrations in the tissues (around 0.10 mg g−1 DM) are not shown for clarity reasons. Means ± SE are shown. | ||
Fv/Fm did not vary significantly during the culture period in the control samples exposed to the P regime, whereas decreased slightly but significantly (P < 0.001) in the PA and PAB regimes, especially on the first few days (Fig. 2). In the Cd-treated samples, the decrease in Fv/Fm was equally significant (P < 0.001) but stronger and more progressive as the experiment progressed, particularly in the PA and PAB regimes.
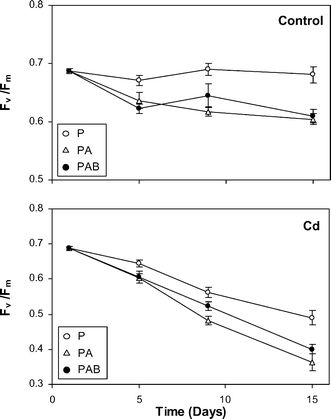 | ||
| Fig. 2 Temporal variations in Fv/Fm, along the culture period, in the liverwort samples cultured in the two culture media (control and Cd) and under the three radiation regimes (P, PA and PAB). Means ± SE are shown. | ||
Table 2 shows the overall effects of the culture medium (control vs. Cd) and radiation regime (P, PA and PAB) on the physiological variables of the liverwort at the end of the experiment. Variables calculated per DM and surface area showed a similar behaviour, since sclerophylly did not change along the culture, and thus effects are expressed only per unit DM for the purpose of brevity. Most of the physiological variables were significantly affected by radiation regime and especially by the culture medium.
| Culture medium | Radiation regime | |
|---|---|---|
| Sclerophylly index (SI) | NS | NS |
| Chlorophyll per DM | *** | *** |
| Chl a/b | *** | NS |
| Carotenoid per DM | *** | NS |
| OD430/OD665 | *** | *** |
| OD430/OD410 | *** | *** |
| Xanthophyll index | ** | *** |
| (A + Z)/Chl a | *** | NS |
| Fv/Fm | ** | * |
| ΦPSII | ** | *** |
| ETRmax | *** | ** |
| NPQmax | *** | NS |
| PN per DM | *** | *** |
| RD per DM | NS | * |
| Trans-phaselic acid per DM | *** | NS |
| Cis-phaselic acid per DM | NS | * |
| Trans-p-coumaroylmalic acid per DM | *** | NS |
| Feruloylmalic acid per DM | *** | *** |
| Coumarin 1 per DM | NS | ** |
| Coumarin 2 per DM | ** | NS |
The initial and final values of the physiological variables in the two culture media and under the three radiation regimes are shown in Fig. 3–5. At the beginning, the samples displayed high vitality as indicated by the high values of chlorophyll concentration, Chl a/b, and Fv/Fm, whereas variables indicative of photoprotection (OD430/OD665, xanthophyll index, NPQmax) were relatively low. In the control plants, PAB samples showed, with respect to P ones, significant decreases in chlorophyll concentration, OD430/OD410, Fv/Fm, phaselic acid (both trans- and cis-) and feruloylmalic acid, and significant increases in OD430/OD665, (A + Z)/Chl a and trans-p-coumaroylmalic acid. DNA damage (thymine dimers) was detected only in PAB samples (see also Fig. 6). The responses of PA samples were similar to those found in the PAB ones in most variables, except for OD430/OD410 and the concentrations of chlorophyll, carotenoid, and trans-p-coumaroylmalic acid, which displayed values significantly different to those of PAB samples and similar to P ones. In the Cd-treated plants, PAB samples showed, with respect to P ones, similar responses to control plants, except for some variables: the concentrations of chlorophyll and feruloylmalic acid, and the OD430/OD665 and (A + Z)/Chl a indices showed similar values in both regimes, Chl a/b decreased in PAB samples and RD rates increased in PAB samples. PAB and PA samples revealed significant differences in more variables than in the control medium, particularly by the higher values in RD, PN, and ΦPSII in the PAB samples than in the PA ones.
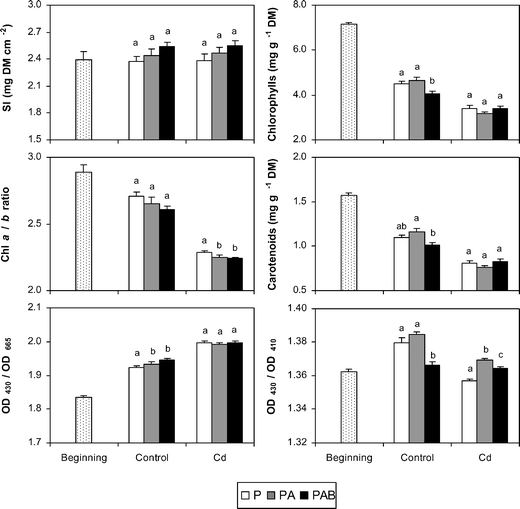 | ||
| Fig. 3 Final values of sclerophylly index (SI), chlorophyll and carotenoid concentrations, Chl a/b ratio, and two pigment indices (OD430/OD665 and OD430/OD410) in the two culture media (control and Cd) and under the three radiation regimes (P, PA and PAB). Different letters indicate significant differences (at least P < 0.05) between the radiation regimes for each culture medium. Initial values of variables are also included. Means ± SE are shown. | ||
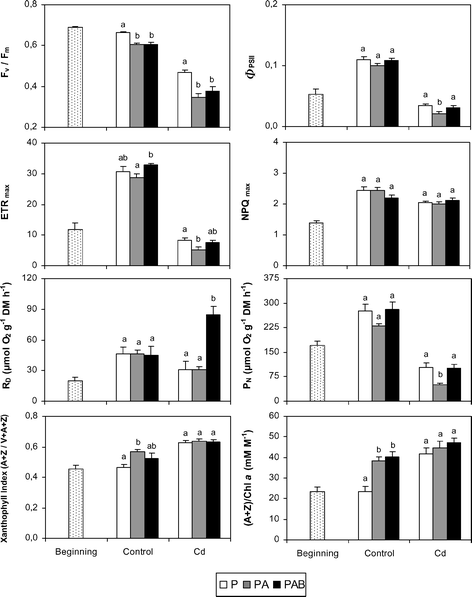 | ||
| Fig. 4 Final values of chlorophyll fluorescence variables, net photosynthesis (PN) and dark respiration (RD) rates, xanthophyll index ((A + Z)/(V + A + Z)) and (A + Z)/Chl a in the two culture media (control and Cd) and under the three radiation regimes (P, PA and PAB). Different letters indicate significant differences (at least P < 0.05) between the radiation regimes for each culture medium. Initial values of variables are also included. Means ± SE are shown. | ||
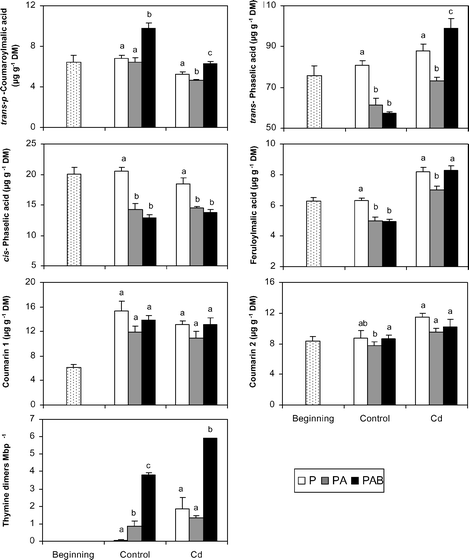 | ||
| Fig. 5 Final values of the concentrations of individual UV-absorbing compounds of J. cordifolia and thymine dimers in the two culture media (control and Cd) and under the three radiation regimes (P, PA and PAB). Coumarins 1 and 2 are named in full in the text. Different letters indicate significant differences (at least P < 0.05) between the radiation regimes for each culture medium. Initial values are also included (thymine dimers were not measured at the beginning of the experiment). Means ± SE are shown. | ||
 | ||
| Fig. 6 Dot blot of DNA from plasmid pBSK and from the liverwort (at the end of the culture period) showing the presence of thymine dimers. Lane a (1–7) corresponds to increasing concentrations (1, 2, 3, 4, 5, 8 and 10 ng) of plasmid DNA irradiated with UV-C (calibration lane). In lane b, columns represent 1 µg samples of liverwort DNA from the Cd medium (column 2, PAB; 3, PA; 4, P) and from the control medium (column 5, PAB; 6, PA; 7, P). Lanes c and d (columns 2–7) are replicates of lane b. In the position c1, a 1 µg sample of non-irradiated plasmid DNA is located. | ||
With notable independence of the radiation regime considered, the Cd-treated samples showed significantly lower values (at least P < 0.05) in diverse vitality variables (chlorophyll and carotenoid concentration, Chl a/b, OD430/OD410, Fv/Fm, ΦPSII, ETRmax and PN) than the control samples, whereas the opposite was observed for OD430/OD665, xanthophyll index, (A + Z)/Chl a, and the concentrations of feruloylmalic acid, trans-phaselic acid and coumarin 2. In the Cd-treated samples, DNA damage was modest in the P and PA samples, and increased outstandingly in the PAB ones.
The first two components of the PCA accounted for 78% of total variance. In the plot generated with the scores of those components (Fig. 7), the initial samples appear in the extreme bottom right of the diagram, clearly separated from the rest. Control samples are grouped in the positive section of both axes, whereas Cd-treated ones appear towards the negative area of the first axis. For both culture media, the samples exposed to the three radiation regimes are neatly separated, with P samples towards the bottom, PAB ones towards the top and PA ones in an intermediate place. For the first axis, the most significant positive loading factors are a compact group of physiological variables indicating vitality (e.g. chlorophyll concentration, Chl a/b, Fv/Fm and PN), while the most significant negative loading factors are some variables indicative of photoprotection (OD430/OD665, xanthophyll index), and the concentrations of feruloylmalic acid and coumarin 2. The most significant loading factors of the second axis are, in the positive part, NPQmax, ETRmax, and the concentration of coumarin 1, and, in the negative part, the concentration of cis-phaselic acid.
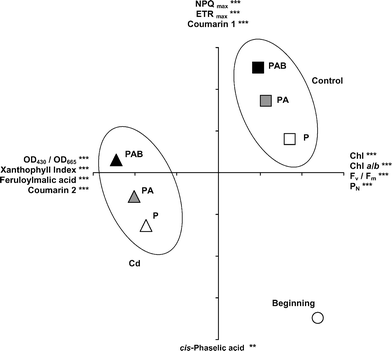 | ||
| Fig. 7 Principal Components Analysis (PCA) performed using the final values of the physiological variables of the samples cultivated in the two culture media (control and Cd) and under the three radiation regimes (P, PA and PAB), together with the initial values (beginning). The most significant loading factors for the positive and negative parts of each axis are shown (*** P < 0.001, ** P < 0.01). Coumarins 1 and 2 are named in full in the text. Axis 1 is the horizontal one and axis 2 the vertical one. Each tick-mark on axes 1 and 2 represents 1 unit. | ||
Discussion
In the Cd-treated culture, J. cordifolia accumulated Cd in a time-dependent manner, which is congruent with the two-compartment (extra- and intracellular) accumulation of Cd in bryophytes.41,42 Given that Cd uptake in bryophytes is mainly passive and extracellular,43,44 it was not influenced by the presence of UV radiation. In the control medium, the Cd concentration in the liverwort was very low, in line with the undetectable Cd concentration in the water.Fv/Fm has been used extensively as a stress index, since it decreases under diverse adverse conditions.45 In this study, Fv/Fm decreased sharply in the Cd-treated samples as compared to controls, as reported elsewhere,16,17 and final values in the former indicated severe damage in the photosynthetic apparatus. In the control plants, Fv/Fm decreased in the samples exposed to UV radiation (PA and PAB regimes), as reported in other similar studies with bryophytes28,32,47–49 and higher plants.50 The decrease observed under the PAB regime was expected because in a previous experiment J. cordifolia behaved as a relatively UV-B-tolerant species but Fv/Fm was the most sensitive variable to UV-B.28 The lowest value of Fv/Fm was found in the plants exposed to both Cd and UV radiation. In Brassica napus, a similar synergistic effect was identified only in Chl a/b and NPQ, when applying comparable Cd concentrations and higher levels of UV-BBE than ours.16 In J. cordifolia, the combined effects of Cd and UV radiation might be more intense than in vascular plants due to the closer contact between the metal and the photosynthetic cells of the liverwort, given that bryophyte leaves are monostratified. Therefore, Fv/Fm was an appropriate variable for establishing the combined stressing effects of Cd and UV radiation under the experimental conditions applied in the present study.
Overall, Cd severely affected almost all the physiological variables, particularly those indicative of plant vitality, such as chlorophyll concentration, Chl a/b, PN, Fv/Fm, ΦPSII, and ETRmax. This was expected in the light of findings reported in other studies conducted on bryophytes, vascular plants, and algae.16,17,22,23,46,51,52 The photosynthetic capacity of the liverwort decreased by around 60% with respect to the controls, which is congruent with the Cd-induced damage on different photosynthetic targets, such as chlorophyll, photosystems II and I, the oxygen-evolving complex, Rubisco and other enzymes of the Calvin cycle.1,46,51 In addition, Cd caused an increase in photoprotection variables, such as OD430/OD665, the xanthophyll index and (A + Z)/Chl a. These responses are usual in photoinhibited plants,53 and the increase in OD430/OD665 and (A + Z)/Chl a could also have been promoted by the dramatic degradation of chlorophyll. No such increase was observed in another photoprotection variable, NPQmax, which may increase16 or decrease17 in vascular plants exposed to similar Cd concentrations. This result can be explained because PSII activity was strongly inhibited by Cd, and under these conditions NPQ does not increase.51 Photoprotective mechanisms could also provide antioxidant molecules to prevent Cd-induced oxidative stress. In this sense, the increase in trans-phaselic acid, feruloylmalic acid, and the coumarin 2, could have benefited the antioxidant capacity of the liverwort, since many phenolic compounds may serve as non-enzymatic antioxidants under heavy metal stress.1,2 Nevertheless, the enhanced production of those compounds did not prevent the severe damage displayed by the Cd-exposed plants. In experiments with Phaseolus coccineus, Cd increased the leaf content of some flavonols,54 but its effect on hydroxycinnamic acid derivatives is known only for pine roots55 and thus comparison with our data is difficult.
Regarding the influence of UV radiation, the control plants under the PAB regime showed, with respect to P ones, significant reductions in chlorophyll concentration, OD430/OD410, and Fv/Fm, but significant increases in OD430/OD665 and (A + Z)/Chl a (probably due to the chlorophyll decrease), reflecting overall lower vitality. These responses may be surprising in such a UV-B tolerant species like J. cordifolia.28,49 However, previous experiments on this species have been conducted under low PAR and the responses to UV radiation depend on the proportions between PAR, UV-A, and UV-B.9 The changes observed were congruent, since chlorophyll and the photosynthetic machinery are recognized targets of UV-B,10 and almost all the cited responses have previously been found in UV-B-sensitive bryophytes, such as the moss Fontinalis antipyretica.28,49 Other physiologically important variables, such as Chl a/b, PN, RD, ΦPSII, ETRmax, NPQmax, and the xanthophyll index responded in the same manner in the P and PAB regimes, suggesting that J. cordifolia was relatively UV-B-tolerant even under the harmful conditions imposed in this study. This tolerance could be based on the global accumulation of UV-absorbing compounds.28,49 Indeed, UV radiation influenced the concentration of several individual compounds. For example, PA and PAB samples had lower levels of phaselic acid (both trans- and cis-) and feruloylmalic acid than P samples, and PAB plants had higher concentrations of trans-p-coumaroylmalic acid than P and PA plants. In contrast, coumarins were hardly affected by the radiation regime.
In the control samples, the responses to PA and PAB regimes were similar in most variables. This may be surprising since UV-A barely had any physiological effects in bryophytes, including J. cordifolia.28,56,57 These discrepancies may be due to different experimental conditions, and could also be related to the recent tendency to consider UV-A when calculating the biologically effective UV radiation (UVBE;5 see Table 1). In this sense, the PA regime in our study had 55% of the UVBE of the PAB regime, and this difference could have some physiological effects. Nevertheless, certain changes only occurred under the specific influence of UV-B, such as the decrease in chlorophyll and OD430/OD410, and the notable increase of trans-p-coumaroylmalic acid. The first two changes are indicative of stress,36 whereas accumulation of trans-p-coumaroylmalic acid could enhance the protective capacity against UV-B. In addition, DNA damage appeared mainly in the PAB samples, which is congruent with data obtained in the moss Sanionia uncinata58,59 and with the target character of DNA with respect to UV-B.10
Only a few studies have considered the interaction between Cd and UV radiation in bryophytes23 and other photosynthetic organisms.16–23 Here, Cd affected a larger number of physiological variables than UV radiation and was a stronger stressing factor, as reported elsewhere.17,20 Physiological damage is usually enhanced by the combination of Cd and UV radiation,18,19,22,23 although this intensification may depend on the variable considered. In J. cordifolia, the stronger influence of Cd perhaps has masked the concomitant effects of UV on many variables, but a certain additiveness between Cd and UV-B could have prompted the increase in DNA damage and OD430/OD665, and the decrease in chlorophyll and carotenoid concentrations, Chl a/b, (A + Z)/Chl a, and especially Fv/Fm. These effects are clearly revealed by the PCA, where the samples are clearly grouped by culture medium and radiation regime, and neatly separated from the initial state. In the light of their respective loading factors, the first axis represents a combined gradient of vitality and photoprotection (more vitality and less photoprotection towards the positive part), whereas the second axis expresses increasing photoprotection towards the positive part. Initial samples possessed great vitality and scarce photoprotection, whereas, at the end of the culture period, the control samples had lost some vitality and had increased photoprotection (maybe due to the high PAR level in the culture), and the Cd-treated samples had a very low vitality and had increased both the photoprotection and the synthesis of feruloylmalic acid, coumarin 2 and cis-phaselic acid (maybe to increase the antioxidant capacity). For both culture media, the PAB samples displayed less vitality and more photoprotection than PA and P ones, so that UV-B wavelengths caused the strongest damage. The additiveness between Cd and UV radiation was to be expected, since both factors may harm plant metabolism through some similar mechanisms, such as the production of reactive oxygen species, DNA damage, and alterations in the photosynthetic machinery.1,2,10,21–23
Finally, the sclerophylly of the samples was not affected by either Cd or UV radiation as expected given that the culture period was too short to allow significant growth. Changes in sclerophylly in J. cordifolia have only been detected in longer culture periods (36–82 d).28,49
Conclusions
Both Cd and UV radiation caused a decrease in chlorophyll concentration and maximum quantum yield of PSII (Fv/Fm) in the aquatic liverwort J. cordifolia, together with an increase in the mechanisms of non-photochemical dissipation of energy (increase in the xanthophyll index). Under the experimental conditions used in the present study, Cd was more stressing than UV radiation, since it had a more severe effect on chlorophyll and Fv/Fm, and it was the only factor influencing negatively photosynthesis in a global manner (decrease in PN, ΦPSII and ETRmax). Given that both Cd and UV may cause concomitant damage in different photosynthetic sites and processes (PSII, the oxygen evolving complex, and the activity of Rubisco and other enzymes of the Calvin cycle),1,10,46,51 the absence of UV radiation effects on global photosynthesis was probably due to the relative UV-tolerance of J. cordifolia.UV radiation increased the levels of trans-p-coumaroylmalic acid and Cd did the same with trans-phaselic acid and feruloylmalic acid in particular. These responses could be related to both, a more efficient absorption of harmful UV radiation and an enhanced protection against oxidative stress. DNA damage was specifically caused by UV-B radiation, whereas Cd itself had a modest effect. Nevertheless, the strongest DNA damage was recorded under the presence of both UV-B and Cd, which could act synergistically through two different mechanisms: UV-B would primarily induce the formation of thymine dimers and Cd would impair the enzymatic repair mechanisms of DNA. UV radiation and Cd seemed to operate additively on some physiological processes, while other responses are probably due to either factor alone.
Acknowledgements
We are grateful to the Ministerio de Educación y Ciencia of Spain and the Fondo Europeo de Desarrollo Regional (FEDER), and to the Gobierno de La Rioja (Consejería de Educación, Cultura, Juventud y Deportes), for their financial support through the Projects REN2002-03438/CLI, CGL2005-02663/BOS and ACPI 2003/06. Saúl Otero and María Arróniz-Crespo benefited from grants of the Ministerio de Educación y Ciencia. Prof. D.-P. Häder (Erlangen) and Prof. O. Nikaido (Kanazawa) are gratefully acknowledged for providing the plasmid pBSK and the primary antibody, respectively. Nacho Esquisábel (Gobierno de La Rioja) kindly authorized our work in the Natural Park of Sierra Cebollera.References
- L. Sanità di Toppi and R. Gabbrielli, Response to cadmium in higher plants, Environ. Exp. Bot., 1999, 41, 105–130 CrossRef.
- P. L. Gratao, A. Polle, P. J. Lea and R. A. Azevedo, Making the life of heavy metal-stressed plants a little easier, Funct. Plant Biol., 2005, 32, 481–494 Search PubMed.
- R. L. McKenzie, L. O. Björn, A. Bais and M. Ilyasd, Changes in biologically active ultraviolet radiation reaching the Earth's surface, Photochem. Photobiol. Sci., 2003, 2, 5–15 RSC.
- M. M. Caldwell, Solar UV irradiation and the growth and development of higher plants, in Photophysiology: current topics in photobiology and photochemistry, ed. A. C. Giese, Academic Press, New York, 1971, vol. 6, pp. 131–177 Search PubMed.
- S. D. Flint and M. M. Caldwell, A biological spectral weighting function for ozone depletion research with higher plants, Physiol. Plant., 2003a, 117, 137–144 Search PubMed.
- S. D. Flint and M. M. Caldwell, Field testing of UV biological spectral weighting functions for higher plants, Physiol. Plant., 2003b, 117, 145–153 Search PubMed.
- S. D. Flint, R. J. Ryel and M. M. Caldwell, Ecosystem UV-B experiments in terrestrial communities: a review of recent findings and methodologies, Agric. Forest Meteorol., 2003, 120, 177–189 Search PubMed.
- S. D. Flint, P. S. Searles and M. M. Caldwell, Field testing of biological spectral weighting functions for induction of UV-absorbing compounds in higher plants, Photochem. Photobiol., 2004, 79, 399–403 CrossRef CAS.
- T. A. Day and P. J. Neale, Effects of UV-B radiation on terrestrial and aquatic primary producers, Ann. Rev. Ecol. Syst., 2002, 33, 371–396 CrossRef.
- M. A. K. Jansen, V. Gaba and B. M. Greenberg, Higher plants and UV-B radiation: balancing damage, repair and acclimation, Trends Plant Sci., 1998, 3, 131–135 CrossRef.
- P. S. Searles, S. D. Flint and M. M. Caldwell, A meta-analysis of plant field studies simulating stratospheric ozone depletion, Oecologia, 2001, 127, 1–10 Search PubMed.
- L. A. Franklin and R. M. Forster, The changing irradiance environment: consequences for marine macrophyte physiology, productivity and ecology, Eur. J. Phycol., 1997, 32, 207–232 Search PubMed.
- R. Sommaruga and F. Garcia-Pichel, UV-absorbing mycosporine-like compounds in planktonic and benthic organisms from a high-mountain lake, Arch. Hydrobiol., 1999, 144, 255–269 Search PubMed.
- D. P. Häder, H. D. Kumar, R. C. Smith and R. C. Worrest, Aquatic ecosystems: effects of solar ultraviolet radiation and interactions with other climatic change factors, Photochem. Photobiol. Sci., 2003, 2, 39–50 RSC.
- V. G. Kakani, K. R. Reddy, D. Zhao and K. Sailaja, Field crop responses to ultraviolet-B radiation: a review, Agric. Forest Meteorol., 2003, 120, 191–218 Search PubMed.
- E. H. Larsson, J. F. Bornman and H. Asp, Influence of UV-B radiation and Cd2+ on chlorophyll fluorescence, growth and nutrient content in Brassica napus, J. Exp. Bot., 1998, 49, 1031–1039 Search PubMed.
- E. H. Larsson, J. F. Bornman and H. Asp, Physiological effects of cadmium and UV-B radiation in phytochelatin-deficient Arabidopsis thaliana, cad1-3, Aust. J. Plant Physiol., 2001, 28, 505–512 Search PubMed.
- U. C. Shukla, P. C. Joshi and P. Kakkar, Synergistic action of ultraviolet-B radiation and cadmium on the growth of wheat seedlings, Ecotoxicol. Environ. Safety, 2002, 51, 90–96 CrossRef CAS.
- U. C. Shukla and P. Kakkar, Effect of dual stress of ultraviolet-B radiation and cadmium on nutrient uptake of wheat seedlings, Commun. Soil Sci. Plant Anal., 2002, 33, 1737–1749 CrossRef CAS.
- N. Atri and L. C. Rai, Differential responses of three cyanobacteria to UV-B and Cd, J. Microbiol. Biotechnol., 2003, 13, 544–551 CAS.
- P. Bhargava, A. K. Srivastava, S. Urmil and L. C. Rai, Phytochelatin plays a role in UV-B tolerance in N-2-fixing cyanobacterium Anabaena doliolum, J. Plant Physiol., 2005, 162, 1220–1225 CrossRef CAS.
- S. M. Prasad and M. Zeeshan, UV-B radiation and cadmium induced changes in growth, photosynthesis, and antioxidant enzymes of cyanobacterium Plectonema boryanum, Biol. Plant., 2005, 49, 229–236 CrossRef CAS.
- S. M. Prasad, R. Dwivedi, M. Zeeshan and R. Singh, UV-B and cadmium induced changes in pigments, photosynthetic electron transport activity, antioxidant levels and antioxidative enzyme activities of Riccia sp., Acta Physiol. Plant., 2004, 26, 423–430 Search PubMed.
- W. B. Bowden, D. Arscott, D. Pappathanasi, J. Finlay, J. M. Glime, J. LaCroix, C. L. Liao, A. Hershey, T. Lampella, B. Peterson, W. Wollheim, K. Slavik, B. Shelley, M. B. Chesterton, J. A. Lachance, R. M. LeBlanc, A. Steinman and A. Suren, Roles of bryophytes in stream ecosystems, J. North Am. Benthol. Soc., 1999, 18, 151–184 Search PubMed.
- C. Ah-Peng and C. Rausch de Traubenberg, Bryophytes aquatiques bioaccumulateurs de polluants et indicateurs écophysiologiques de stress: synthèse bibliographique, Cryptog. Bryol., 2004, 25, 205–248 Search PubMed.
- C. Gehrke, Impacts of enhanced ultraviolet-B radiation on mosses in a subarctic heath ecosystem, Ecology, 1999, 80, 1844–1851 Search PubMed.
- S. Huttunen, T. Taipale, N. M. Lappalainen, E. Kubin, K. Lakkala and J. Kaurola, Environmental specimen bank samples of Pleurozium schreberi and Hylocomium splendens as indicators of the radiation environment at the surface, Environ. Pollut., 2005, 133, 315–326 CrossRef CAS.
- J. Martínez-Abaigar, E. Núñez-Olivera, N. Beaucourt, M. A. García-Álvaro, R. Tomás and M. Arróniz, Different physiological responses of two aquatic bryophytes to enhanced ultraviolet-B radiation, J. Bryol., 2003, 25, 17–30 Search PubMed.
- K. K. Newsham, D. A. Hodgson, A. W. A. Murray, H. J. Peat and R. I. Lewis Smith, Response of two Antarctic bryophytes to stratospheric ozone depletion, Global Change Biol., 2002, 8, 972–983 Search PubMed.
- S. A. Robinson, J. D. Turnbull and C. E. Lovelock, Impact of changes in natural ultraviolet radiation on pigment composition, physiological and morphological characteristics of the Antarctic moss, Grimmia antarctici, Global Change Biol., 2005, 11, 476–489 Search PubMed.
- P. S. Searles, S. D. Flint, S. B. Díaz, M. C. Rousseaux, C. L. Ballaré and M. M. Caldwell, Solar ultraviolet-B radiation influence on Sphagnum bog and Carex fen ecosystems: first field season findings in Tierra del Fuego, Argentina, Global Change Biol., 1999, 5, 225–234 Search PubMed.
- Z. Takács, Z. Csintalan, L. Sass, E. Laitat, I. Vass and Z. Tuba, UV-B tolerance of bryophyte species with different degrees of desiccation tolerance, J. Photochem. Photobiol., B, 1999, 48, 210–215 CrossRef CAS.
- M. Arróniz-Crespo, Efectos de la radiación ultravioleta-B sobre briófitos acuáticos de ríos de montaña, PhD Thesis, Universidad de La Rioja, Logroño, Spain, 2005 Search PubMed.
- R. B. Setlow, The wavelengths in sunlight effective in producing skin cancer: a theoretical analysis, Proc. Natl. Acad. Sci. U. S. A., 1974, 71, 3363–3366 CAS.
- L. O. Björn and A. H. Teramura, Simulation of daylight ultraviolet radiation and effects of ozone depletion, in Environmental UV Photobiology, ed. A. R. Young, Plenum Press, New York, 1993, pp. 41–71 Search PubMed.
- J. Martínez-Abaigar and E. Núñez-Olivera, Ecophysiology of photosynthetic pigments in aquatic bryophytes, in Bryology for the Twenty-first Century, ed. J. W. Bates, N. W. Ashton and J. G. Duckett, Maney Publishing and the British Bryological Society, Leeds, 1998, pp. 277–292 Search PubMed.
- J. De Las Rivas, A. Abadía and J. Abadía, A new reversed phase-HPLC method resolving all major higher plant photosynthetic pigments, Plant Physiol., 1989, 91, 190–192 CrossRef CAS.
- U. Schreiber, W. Bilger and C. Neubauer, Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis, in Ecophysiology of photosynthesis, ed. E. D. Schulze and M. M. Caldwell, Springer, Berlin, 1995, pp. 49–75 Search PubMed.
- P. G. Falkowski and J. A. Raven, Aquatic Photosynthesis, Blackwell Science, Oxford, 1997, p. 375 Search PubMed.
- R. P. Sinha, M. Dautz and D. P. Häder, A simple and efficient method for the quantitative analysis of thymine dimers in cyanobacteria, phytoplankton and macroalgae, Acta Protozool., 2001, 40, 187–195 CAS.
- L. Croisetiere, L. Hare, A. Tessier and S. Duchesne, Modeling cadmium exchange by an aquatic moss (Fontinalis dalecarlica), Environ. Sci. Technol., 2005, 39, 3056–3060 CrossRef CAS.
- M. D. Vázquez, J. López and A. Carballeira, Uptake of heavy metals to the extracellular and intracellular compartments in three species of aquatic bryophytes, Ecotoxicol. Environ. Safety Environ. Res. B, 1999, 44, 12–24 Search PubMed.
- C. Bleuel, D. Wesenberg, K. Sutter, J. Miersch, B. Braha, F. Barlocher and G. Krauss, The use of the aquatic moss Fontinalis antipyretica L. ex Hedw. as a bioindicator for heavy metals - 3. Cd2+ accumulation capacities and biochemical stress response of two Fontinalis species, Sci. Total Environ., 2005, 345, 13–21 CrossRef CAS.
- I. Bruns, K. Sutter, S. Menge, D. Neumann and G. J. Krauss, Cadmium lets increase the glutathione pool in bryophytes, J. Plant Physiol., 2001, 158, 79–89 CrossRef CAS.
- K. Maxwell and G. N. Johnson, Chlorophyll fluorescence—a practical guide, J. Exp. Bot., 2000, 51, 659–668 Search PubMed.
- M. K. Joshi and P. Mohanty, Chlorophyll a fluorescence as a probe of heavy metal ion toxicity in plants, in Chlorophyll a fluorescence: A signature of photosynthesis, ed. G. C. Papageorgiou and Govindjee, Springer, Dordrecht, 2004, pp. 637–661 Search PubMed.
- C. Ihle and H. Laasch, Inhibition of photosystem II by UV-B radiation and the conditions for recovery in the liverwort Conocephalum conicum Dum., Bot. Acta, 1996, 109, 199–205 Search PubMed.
- P. Montiel, A. Smith and D. Keiller, Photosynthetic responses of selected Antarctic plants to solar radiation in the southern maritime Antarctic, Polar Res., 1999, 18, 229–235 Search PubMed.
- E. Núñez-Olivera, J. Martínez-Abaigar, R. Tomás, N. Beaucourt and M. Arróniz-Crespo, Influence of temperature on the effects of artificially enhanced UV-B radiation on aquatic bryophytes under laboratory conditions, Photosynthetica, 2004, 42, 201–212 CrossRef CAS.
- B. R. Jordan, The effects of ultraviolet-B radiation on plants: a molecular perspective, Adv. Bot. Res., 1996, 22, 97–162 Search PubMed.
- R. Popovic, D. Dewez and P. Juneau, Applications of chlorophyll fluorescence in ecotoxicology: heavy metals, herbicides, and air pollutants, in Practical applications of chlorophyll fluorescence in plant biology, ed. J. R. DeEll and P. M. A. Toivonen, Kluwer, Boston, 2003, pp. 151–184 Search PubMed.
- M. Bertrand and I. Poirier, Photosynthetic organisms and excess of metals, Photosynthetica, 2005, 43, 345–353 CrossRef CAS.
- B. Demmig-Adams and W. W. Adams, III, Xanthophyll cycle and light stress in nature: uniform response to excess direct sunlight among higher plant species, Planta, 1996, 198, 460–470 CrossRef CAS.
- E. Skorzynska-Polit, M. Drazkiewicz, D. Wianowska, W. Maksymiec, A. L. Dawidowicz and A. Tukiendorf, The influence of heavy metal stress on the level of some flavonols in the primary leaves of Phaseolus coccineus, Acta Physiol. Plant., 2004, 26, 247–254 Search PubMed.
- A. Schützendübel and A. Polle, Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization, J. Exp. Bot., 2002, 53, 1351–1365 Search PubMed.
- R. Niemi, P. J. Martikainen, J. Silvola, E. Sonninen, A. Wulff and T. Holopainen, Responses of two Sphagnum moss species and Eriophorum vaginatum to enhanced UV-B in a summer of low UV intensity, New Phytol., 2002a, 156, 509–515 Search PubMed.
- R. Niemi, P. J. Martikainen, J. Silvola, A. Wulff, S. Turtola and T. Holopainen, Elevated UV-B radiation alters fluxes of methane and carbon dioxide in peatland microcosms, Global Change Biol., 2002b, 8, 361–371 Search PubMed.
- D. Lud, T. C. W. Moerdijk, W. H. Van de Poll, A. G. J. Buma and A. H. L. Huiskes, DNA damage and photosynthesis in Antarctic and Arctic Sanionia uncinata (Hedw.) Loeske under ambient and enhanced levels of UV-B radiation, Plant, Cell Environ., 2002, 25, 1579–1589 CrossRef CAS.
- D. Lud, M. Schlensog, B. Schroeter and A. H. L. Huiskes, The influence of UV-B radiation on light-dependent photosynthetic performance in Sanionia uncinata (Hedw.) Loeske in Antarctica, Polar Biol., 2003, 26, 225–232 Search PubMed.
- A. Gröniger, C. Hallier and D. P. Häder, Influence of UV radiation and visible light on Porphyra umbilicalis: photoinhibition and MAA concentration, J. Appl. Phycol., 1999, 11, 437–445 Search PubMed.
| This journal is © The Royal Society of Chemistry and Owner Societies 2006 |
