The doping mechanism of Cr into TiO2 and its influence on the photocatalytic performance
Abstract
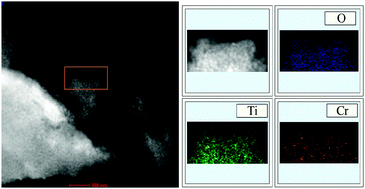
The chromium doped titanium dioxide (Cr–TiO2) has been synthesized using a hydrothermal method. The as-prepared samples have been characterized by X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD), transmission electron microscopy (TEM), high resolution TEM (HR-TEM), XPS valence band spectroscopy, UV-vis diffuse reflectance spectroscopy (UV-vis DR), photoluminescence (PL) spectroscopy and time resolved PL (TR-PL) spectroscopy. The doping mechanism and related influence on the photocatalytic performance of TiO2 are thus proposed. The doped Cr3+ ions can replace the Ti atoms in the lattice with oxygen vacancy compensation, distribute homogeneously in the framework of TiO2 crystals, and may make the n-type TiO2 less n-type or more p-type due to the resultant formation of oxygen vacancies, resulting in absorption of visible light, decrease of the intensity of PL emission and prolonged lifetime of photogenerated charge carriers. Compared with TiO2, the doped samples exhibit an improved visible-light photocatalytic activity. The influence of nitrogen modification has also been studied. We envision that these results would afford a better understanding of the doping mechanism of TiO2 using metal ions and, therefore, may provide a feasible way to prepare the TiO2-based photocatalysts for real applications.

 Please wait while we load your content...
Please wait while we load your content...