Conformational communication between the Ar–CO and Ar–N axes in 2,2′-disubstituted benzanilides and their derivatives
Jonathan
Clayden
*,
Lluís
Vallverdú
and
Madeleine
Helliwell
School of Chemistry, University of Manchester, Oxford Road, Manchester, UK M13 9PL. E-mail: clayden@man.ac.uk; Fax: +44 (0) 161 275 4612
First published on 25th April 2006
Abstract
Benzanilides containing two or more potentially stereogenic amide axes exist in solution as mixtures of conformers which are detectable by NMR. For simple tertiary benzanilides carrying an ortho substituent on each ring, conformational control can be high (up to about 10 : 1) providing the substituents are large, indicating that the two axes are in conformational communication with one another. For more complex diamides, conformational communication breaks down, and mixtures of conformers are evident by NMR.
Introduction
Over the last few years there has been a growing interest in the preparation of non-biaryl atropisomers.1,2 Because of the rigidity of the amide group, the most studied of these non-biaryl atropisomers have been either benzamide derivatives 1 or anilides 2 (Fig. 1). Our own work has concentrated primarily on ortho-substituted tertiary benzamides 1,3 in which the amide group lies more or less perpendicular to the plane of the aromatic ring.3c Provided rotation about the Ar–CO bond is slow enough, which is usually the case with 2,6-disubstituted benzamides (1: X, Y ≠ H), atropisomeric chirality results from this perpendicular arrangement. Even for less hindered compounds (mono-ortho-substituted benzamides for example, 1: X or Y = H), transient chirality (on the NMR timescale) manifests itself spectroscopically in the diastereotopicity of groups attached to the ring or the amide nitrogen atom.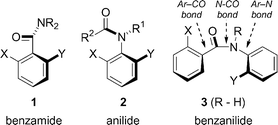 | ||
| Fig. 1 Benzamides, anilides and benzanilides. | ||
Axial chirality in ortho-substituted anilides 2 was recognized more than fifty years ago.4,5 While differentially N,N-disubstituted amides generally prefer to exist as Z-rotamers (i.e. largest group cis to the oxygen) for steric reasons, it has been known for some time that N-alkyl acetanilides 2 (R2 = Me)6 and many N-alkyl benzanilides 2 (R2 = Ar)7 prefer to exist as the E-rotamer, which places the aryl group and the amide oxygen in the trans conformation, as shown for 2. In general, atropisomerism is observed when an anilide carries two different non-hydrogen ortho-substituents X and Y. A large number of anilides with axial chirality have been described,8 and we have recently reported that mono-ortho-substituted anilides may also exhibit chirality provided that the ortho substituent is iodo.9
We now report the preparation and conformational studies of compounds combining the amide structural motifs of both 1 and 2—2,2′-disubstituted benzanilides 3 (Fig. 1). Using NMR, we demonstrate a degree of conformational interaction between the Ar–CO and N–CO axes, suggesting their potential application in stereochemical relay systems.10
Results and discussion
Conformational ratios in benzanilides
Compounds 3a–o were prepared by the standard method shown in Scheme 1: available anilines and benzoyl chlorides were condensed to yield secondary anilides 4 (R = H). Alkylation of these compounds gave tertiary benzanilides 3a–o, whose 1H-NMR spectra at 25 °C in CDCl3 show that they exist as a mixture of conformers about two or more of the Ar–CO, Ar–N and N–CO bonds in solution. All of these bonds are expected to rotate slowly on the NMR timescale at 25 °C, though fast on the laboratory timescale: typical barriers to rotation of such bonds in similar compounds lie in the region of 60–80 kJ mol−1.3h,9Table 1 shows the ratios of the two or more conformers quantified by integration of the paired signals.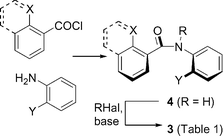 | ||
| Scheme 1 Synthesis of 2,2′-disubstituted benzanilides. | ||
| Compound | X | Y | R | Conformer ratioa |
|---|---|---|---|---|
| a Ratio in CDCl3 at 25 °C. b 1-naphthamide. | ||||
| 3a | I | I | Bn | 10 : 1 |
| 3b | I | I | o-I–Bn | 16 : 1.2 : 1 |
| 3c | I | I | Et | 4.3 : 1 |
| 3d | I | I | i-Pr | 2.5 : 1 |
| 3e | Benzob | I | Bn | 7 : 1 |
| 3f | Benzob | CO2Et | Bn | 5 : 1 |
| 3g | Benzob | CO2H | Bn | 3.25 : 1 |
| 3h | I | CO2Et | Bn | 5.3 : 1 |
| 3i | I | CO2H | Bn | 3.5 : 1 |
| 3j | NO2 | I | Bn | 12 : 1 |
| 3k | NO2 | I | Et | 6.4 : 1 |
| 3l | NO2 | I | Me | 3.6 : 1 |
| 3m | NH2 | I | Bn | Broad |
| 3n | NH2 | I | Et | Broad |
| 3o | NH2 | I | Me | Broad |
The degree of conformational control is governed by the size of the substituents at the ortho positions (X and Y) and also the substituent at the nitrogen (R): the bulkier they are, the higher the conformational ratio. In 3a (X = Y = I, R = Bn) and 3j (X = NO2, Y = I, R = Bn) the ratios reach a remarkable 10 : 1 and 12 : 1 respectively. Addition of a third bulky ortho-substituent, i.e. an extra iodo group at the ortho position of the N-benzyl group, adds a third conformer to the mixture: 3b displays a conformational ratio of 16 : 1.2 : 1. The ratio between the two major conformers is nonetheless similar to the one observed for 3j.
As the size of any of the substituents (X, Y or R) decreases, the conformational ratio also drops. For example, taking 3a as a reference with X = Y = I and R = Bn, a reduction in size of any of the three groups shows lower ratios, as can be observed in 3c and 3d with a smaller R (Et, i-Pr), 3e with a smaller X, and 3f and 3g with smaller Y groups.
The broad signals in the NMR spectra of 3m–o indicate fast interconversion of the possible conformations, making determination of the conformational ratio impossible. In contrast, spectra of the analogous compounds with a NO2 group, 3j–l, have sharp signals characteristic of slow exchange. Fast Ar–CO rotation in 3m–o is most likely due to stabilisation of the planar transition state for Ar–CO rotation by the electron-donating amino group.3h
Identification of the conformers
The X-ray crystal structures of 3a and 3b showed the expected E-rotamer6,7 about the N–CO bond in the solid state, with the two iodo substituents on opposite faces of the plane of the amide, presumably in order to avoid steric interactions (Fig. 2 and 3). We confirmed that this E-anti conformer observed in the crystal structure is also the major conformer in solution by dissolving a crystal of 3a in cold CDCl3. The 1H-NMR spectrum of this solution at low temperature showed a single conformer corresponding to the major conformer in the spectrum at room temperature. Allowing the solution to warm restored the 10 : 1 ratio. By contrast, a sample prepared at room temperature and cooled to −50 °C showed a mixture of conformers throughout.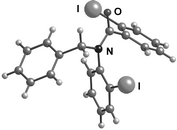 | ||
| Fig. 2 X-Ray structure of 3a. | ||
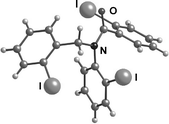 | ||
| Fig. 3 X-Ray structure of 3b. | ||
Scheme 2 shows the four possible conformations of 3 that result from rotation about the Ar–N, Ar–CO and N–CO bonds, starting from the E-anti conformer. Molecular mechanics calculations (MMFF force field implemented by Spartan) for 3a (X = Y = I; R = Bn) are in agreement with assignment of the E-anti conformation to the lowest energy conformer. The calculations also indicate that both E rotamers (syn and anti) have lower energy levels than the two Z rotamers. From this analysis we assume that the minor conformers observed by 1H-NMR are the E-syn conformers, and that the ratios in Table 1 represent the ratio of E-anti-3 to E-syn-3, with the exception of 3b, in which another conformer is evident, probably a Z rotamer about the amide N–CO bond.
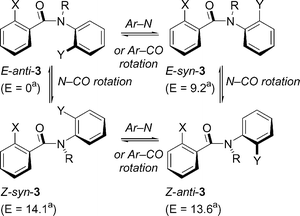 | ||
| Scheme 2 Rotational interconversions in 3. (a) Energy calculated (MMFF, Spartan) in kJ mol−1 relative to E-anti-3 for X = Y = I; R = Bn. | ||
Variable temperature NMR studies of 3a, 3b and 3c were carried out. 3a and 3b showed no change in their spectra over the range −50 to +50 °C in CDCl3 or at +90 °C in d6-DMSO, indicating that all three pertinent bond rotations have barriers of >75.1 kJ mol−1. In contrast, VT NMR studies of 3c in toluene (−70 to +100 °C) showed coalescence of the signals due to the two conformers of 3c, allowing us to estimate a barrier to rotation for either the Ar–CO or Ar–N bond (whichever is the lower) of only 63.5 kJ mol−1.
Conformational ratios in diamides
The high levels of conformational control in certain benzanilides 3 indicate that there is some degree of conformational communication between the Ar–CO and the N–CO axes, provided that the rings bear sufficiently large substituents. We recently reported a case in which conformational communication through a series of six Ar–CO axes facilitated the remote transmission of stereochemical information,11 and with the aim of developing a similar system based on benzanilides we set about the synthesis of the diamides 5. We were encouraged in this aim by reports that N-alkylated oligo(p-benzamides)12 and oligo(m-benzamides)13 adopt a well-defined, helical secondary structure.Our initial attempts to prepare diamides 5 from carboxylic acid derivatives of 3 (3g for example) via the acyl chloride led instead to the oxazinones 6 by cyclisation (Scheme 3). However, compounds 5a–o were eventually successfully prepared by acylation of the amino derivatives 3m–o, themselves produced by reduction of the nitro compounds 3j–l (Scheme 3).
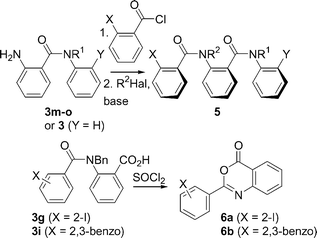 | ||
| Scheme 3 Synthesis of the diamides 5. | ||
Table 2 shows the conformational ratios observed by 1H-NMR at rt in CDCl3 for compounds 5a–o. Compounds 5a, 5d, 5f, 5h, 5k and 5n, with R2 = H, are comparable conformationally to compounds 3 (Table 1) because a secondary amide cannot generate a stereogenic Ar–CO axis and will exist as the Z amide rotamer (Ar and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O cis). These compounds display ratios from 22 : 1 to 5.5 : 1, depending on X, Y and R1, suggesting that the benzoylamino groups allow good levels of stereocommunication between the two remaining axes in the molecule.11
O cis). These compounds display ratios from 22 : 1 to 5.5 : 1, depending on X, Y and R1, suggesting that the benzoylamino groups allow good levels of stereocommunication between the two remaining axes in the molecule.11
| Compound | X | Y | R1 | R2 | Ratio |
|---|---|---|---|---|---|
| 5a | I | I | Bn | H | 12 : 1 |
| 5b | I | I | Bn | CH3 | 6.8 : 2 : 1 |
| 5c | I | I | Bn | Bn | Complex mixture |
| 5d | NO2 | I | Bn | H | 22 : 1 |
| 5e | NO2 | I | Bn | CH3 | 4.5 : 1 : 1 |
| 5f | NO2 | I | CH3 | H | 6.5 : 1 |
| 5g | NO2 | I | CH3 | Et | 3.2 : 2.5 : 1.5 : 1 |
| 5h | NO2 | I | Et | H | 5.5 : 1 |
| 5i | NO2 | I | Et | CH3 | 4.5 : 2 : 1 |
| 5j | NO2 | I | CH3 | CH3 | 4.2 : 4 : 1 |
| 5k | H | I | CH3 | H | 4.5 : 1 |
| 5l | H | I | CH3 | Bn | 1.33 : 1 : ? |
| 5m | H | I | CH3 | Et | 2 : 1 : ? |
| 5n | H | H | Bn | H | 8 : 1 |
| 5o | H | H | Bn | Bn | Broad |
Alkylation of the second amide group (i.e. introduction of R2 ≠ H) introduces a third (and if X ≠ H, a fourth) potentially stereogenic axis to the molecule. Unfortunately, in all such cases, complex mixtures of conformers were observed: the two amide Ar–CO axes fail to interact with each other either directly or via the Ar–N axis. In the majority of the compounds the ratio between the conformers can be established by the integration of the signals corresponding to each conformer. However this becomes impossible in some cases—for example 5c has a 1H-NMR spectrum too complex for resolution of the signals of the individual conformers. In other cases, e.g.5l and 5m, it is possible to identify the ratio of only the two major conformers (by integration of the signal corresponding to the methyl group). Other partly overlapping signals suggest that other conformers are populated, but in ratios that cannot be determined. In the absence of any ortho substituents, i.e.5o, faster interconversion between the conformers leads to broad signals.
Molecular mechanic calculations (Spartan MMFF) suggest that in benzanilides with a bulky ortho substituent (here NR2COAr) on the benzamide ring the Z rotamer becomes preferred to the E rotamer,7 thus placing one amide group of 5 in the E conformation and the other in the Z (Fig. 4). Even with N–CO bonds orientated in this way, four or eight conformers can exist, and the differences in energy between them are minimal.
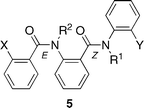 | ||
| Fig. 4 Conformation of the diamide 5. | ||
Conformational communication between benzamide and anilide-type amide axes
With our conformational analysis of compounds 3 and 5 we have probed the ability of a pair of amide axes connected either as shown in Fig. 5A or Fig. 5B to communicate with one another: the results suggest that communication can be successful (subject to the size of the substituents) for A but is not successful for B. Compounds of type C, which contain two adjacent benzamide-type amide axes, are known in many cases to display high levels of conformational control,3f,3t,14 and a detailed publication presenting out findings in this area is in preparation.15 A final pairing—bis-anilides of type D—was investigated by Cheng et al.16 but with tertiary amide groups 7d exists as a mixture of conformers in solution.17 In the solid state the typical7E-rotamer about the N–CO bond (Ar and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O trans) is observed, with the two anilide groups disposed anti. In the crystal structure of 7e, however, in which the ortho positions carry a fluorine atom, while the anilides are still anti, the unexpected opposite Z N–CO rotamer is populated,7,18 an effect possibly related to the calculated switch from the E to the Z conformation in one of the amide bonds of 5.
O trans) is observed, with the two anilide groups disposed anti. In the crystal structure of 7e, however, in which the ortho positions carry a fluorine atom, while the anilides are still anti, the unexpected opposite Z N–CO rotamer is populated,7,18 an effect possibly related to the calculated switch from the E to the Z conformation in one of the amide bonds of 5.
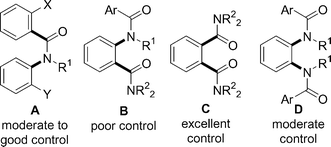 | ||
| Fig. 5 Conformational communication between pairs of amide axes (axes are represented by bold bonds). | ||
We prepared 7b and 7c (Scheme 4) with ortho-iodo substituents in order to slow the rotation about the bonds associated with the amide groups with the aim of establishing the conformational ratios in such compounds in solution. The 1H-NMR spectrum showed a simple 5 : 1 ratio between only two conformers of 7b at rt in CDCl3. In the solid state, 7b adopted a conformation with both N–CO bonds Z and the anilides anti, comparable to that reported for 7e18—the X-ray crystal structure of 7b is shown in Fig. 6. We assume that this is the major conformer in solution, but we have no evidence to allow assignment of the conformation of the minor conformer. It is notable that each crystal of 7b contains only one enantiomeric conformer—the crystals have a chiral space group: monoclinic P2(1).19 An analogue, 7c, carrying N benzyl groups, showed only broad signals in its 1H-NMR spectrum.
 | ||
| Scheme 4 Synthesis of the diamides 7. | ||
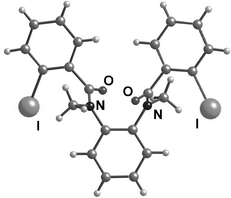 | ||
| Fig. 6 X-Ray crystal structure of 7b. | ||
Conclusion
Conformational analysis of representative members of three classes of aromatic amides and diamides—compounds 3a–o for those represented as Fig. 5A, 5a–n, for those represented as Fig. 5B, and 7b for those represented as Fig. 5D—shows that for compounds of types A and D moderate to high levels of conformational control can be achieved when the amides carry bulky substituents. Type B compounds 5 with two tertiary amide groups exist as complex conformational mixtures whereas the analogous compounds with meta13 and para12 substituted amides exist largely as single conformers. Poor control in this situation may be due to population of the usually disfavoured (for anilides) Z N–CO conformer, a feature also seen in 7. Type C compounds, as previously reported,3f,3h,3t show excellent conformational control in solution, and a detailed report concerning this class of compounds is in preparation.15Experimental
1H and 13C-NMR spectra were recorded on a Varian XL 300 MHz or Bruker Ultrashield 500 MHz instrument. VT NMR studies were recorded on an Inova 300 MHz instrument. NMR data are presented as follows: chemical shift δ (in ppm relative to δTMS = 0), multiplicity, coupling constant J (quoted in hertz, Hz), integration, and assignment. IR spectra were recorded on an ATi Matson Genesis Series Fourier Transform spectrometer. Wavelengths of maximum absorbance are quoted in wavenumbers (cm−1). Low resolution mass spectra (EI and CI) were recorded on a Fisons VG Trio 2000 quadrupole mass spectrometer. High resolution mass spectra were recorded on a Kratos Concept-IS mass spectrometer. Analytical TLC was carried out on Machery-Nagel pre-coated 0.2 mm silica plates with fluorescent indicator on aluminium. Flash column chromatography was carried out using Fluorochem Davisil 40–63 µm 60 A silica under a positive pressure of air. Conformational ratios were calculated by integration of the NMR signals corresponding to the same proton(s) relating to each conformer. When only two conformers are observed they are referred to as major and minor conformers, when more than two are observed they are referred to as conformers A, B, C… listed in decreasing ratio. The purity of compounds 5b, 5c, 5e, 5g, 5l, 5m, 5o and 7b was confirmed by HPLC (Supelcosil LC 18 DB 4.6 mm × 250 mm, 90 : 10 hexane–isopropanol, flow 0.5 mL min−1).2-Iodo-N-(2-iodophenyl)benzamide 4a
To a solution of 2-iodoaniline (1 g, 4.56 mmol) and triethylamine (3.2 ml, 22.9 mmol) in ethyl acetate (25 mL) was added a solution of 2-iodobenzoyl chloride (1.22 g, 4.58 mmol) in ethyl acetate (25 mL). The mixture was stirred at rt under nitrogen overnight. The resulting suspension was filtered and the solid was washed with ether and NaHCO3 aqueous saturated solution to yield the title compound as a white solid (1.1 g, 53%). 1H-NMR (CDCl3, 300 MHz) δ 6.91 (td, J = 7.8 and 1.5 Hz, 1H), 7.18 (td, J = 7.8 and 1.5 Hz, 1H), 7.41 (d, J = 7.5 Hz, 1H), 7.47 (t, J = 7.5 Hz, 1H), 7.58 (d, J = 7.5 Hz, 1H), 7.75 (bs, 1H), 7.83 (dd, J = 8.1 and 1.5 Hz, 1H), 7.97 (dd, J = 8.1 and 1 Hz, 1H), 7.97 (d, J = 8.1 Hz, 1H). 13C-NMR (CDCl3, 75.5 MHz) δ 90.3 (Cq), 92.5 (Cq), 122.3 (CH), 126.6 (CH), 128.1 (CH), 128.4 (CH), 129.4 (CH), 131.7 (CH), 137.9 (Cq), 138.9 (CH), 140.4 (CH), 141.6 (Cq), 167.2 (Cq). IR (film) 3245, 1650 cm−1. HRMS calcd for C13H9ONI2: 448.8768. Found: 448.8776. Anal. calcd for C13H9NOI2 (449.0)·1/4 H2O: C, 34.43; H, 2.11; N, 3.09. Found: C, 34.18; H, 1.87; N, 2.99%. Mp 184–185 °C.N-(2-Iodophenyl)-1-naphthamide 4b
To a solution of 2-iodoaniline (1 g, 4.56 mmol) and triethylamine (3.2 ml, 22.9 mmol) in ethyl acetate (50 mL) was slowly added 1-naphthoyl chloride (0.7 mL, 4.64 mmol). The mixture was stirred overnight at rt under nitrogen. The resulting suspension was filtered and the solid was washed with ether and NaHCO3 aqueous saturated solution to yield the title compound as a yellow solid (909 mg, 53%). 1H-NMR (CDCl3, 300 MHz) δ 6.92 (ddd, J = 7.8, 7.5 and 1.5 Hz, 1H), 7.45 (td, J = 8.4 and 1.2 Hz, 1H), 7.53–7.64 (m, 3H), 7.84 (dd, J = 7.8 and 1.5 Hz, 1H), 7.87 (dd, J = 8.4 and 1.2 Hz, 1H), 7.92 (dd, J = 7.5 and 1.8 Hz, 1H), 8.01 (d, J = 8.4 Hz, 1H), 8.08 (bs, 1H), 8.48–8.54 (m, 2H). 13C-NMR (CDCl3, 75.5 MHz) δ 90.7 (Cq), 122.1 (CH), 124.8 (CH), 125.3 (CH), 125.4 (CH), 126.3 (CH), 126.7 (CH), 127.5 (CH), 128.4 (CH), 129.4 (CH), 130.2 (Cq), 131.5 (CH), 133.8 (Cq), 133.9 (Cq), 138.5 (Cq), 138.9 (CH), 167.4 (Cq). IR (film) 3248 and 1648 cm−1. HRMS calcd for C17H13NOI: 374.0036. Found: 374.0044. Anal. calcd for C17H12NOI (373.2): C, 54.71; H, 3.24; N, 3.75. Found: C, 54.71; H, 3.14; N, 3.68%. Mp 160–161 °C.Ethyl 2-(1-naphthamido)benzoate 4c
To a solution of 2-ethoxycarbonilaniline (1 g, 6.05 mmol) and triethylamine (4.5 mL, 32.3 mmol) in ethyl acetate (50 mL) was slowly added 1-naphthoyl chloride (0.91 mL, 6.04 mmol). The mixture was stirred overnight at rt under nitrogen. The mixture was diluted with ethyl acetate and washed with water. The aqueous layer was extracted with ethyl acetate and the combined organic layers were dried, filtered and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (DCM) to yield 4c (1.14 g, 58%). 1H-NMR (CDCl3, 300 MHz) δ 1.37 (t, J = 7.2 Hz, 3H), 4.33 (q, J = 7.2 Hz, 2H), 7.17 (ddd, J = 8.1, 7.2 and 1.2 Hz, 1H), 7.53–7.61 (m, 3H), 7.66 (td, J = 7.2 and 1.5 Hz, 1H), 7.88 (dd, J = 7.2 and 1.2 Hz, 1H), 7.90 (dd, J = 7.8 and 1.2 Hz, 1H), 7.98 (d, J = 8.4 Hz, 1H), 8.12 (dd, J = 8.1 and 1.8 Hz, 1H), 8.54 (dd, J = 8.1 and 1.5 Hz, 1H), 9.03 (dd, J = 8.4 and 0.9 Hz, 1H), 11.74 (bs, 1H). 13C-NMR (CDCl3, 75.5 MHz) δ 14.1 (CH3), 61.4 (CH2), 115.6 (Cq), 120.5 (CH), 122.8 (CH), 124.9 (CH), 125.4 (CH), 125.6 (CH), 126.4 (CH), 127.2 (CH), 128.3 (CH), 130.4 (Cq), 130.9 (CH), 131.3 (CH), 133.9 (Cq), 134.5 (Cq), 134.6 (CH), 141.7 (Cq), 167.9 (Cq), 168.2 (Cq). IR (film) 3261, 1675 cm−1. HRMS calcd for C20H18NO3: 320.1281. Found: 320.1281. Anal. calcd for C20H17NO3 (319): C, 75.22; H, 5.37; N, 4.39. Found: C, 74.79; H, 5.39; N, 4.35%. Mp 83–84 °C.Ethyl 2-(2-iodobenzamido)benzoate 4d
To a solution of 2-iodobenzoylchloride (1.63 g, 6.12 mmol) and triethylamine (4.2 mL, 30.1 mmol) in ethyl acetate (50 mL) was added ethyl 2-aminobenzoate (0.9 mL, 6.09 mmol), and the mixture was stirred overnight at rt under nitrogen. The mixture was basified with NaHCO3 aqueous saturated solution and extracted with DCM. The organic layer was dried and filtered and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (DCM) to yield 4d (1.94 g, 80%). 1H-NMR (CDCl3, 300 MHz) δ 1.43 (t, J = 7.2 Hz, 3H), 4.39 (q, J = 7.2 Hz, 2H), 7.18 (td, J = 7.5 and 1.8 Hz, 1H), 7.19 (td, J = 7.5 and 1.2 Hz, 1H), 7.48 (td, J = 7.5 and 1.2 Hz, 1H), 7.60 (dd, J = 7.8 and 1.8 Hz, 1H), 7.67 (dd, J = 7.5 and 1.5 Hz, 1H), 7.98 (dd, J = 8.1 and 1.2 Hz, 1H), 8.13 (dd, J = 8.1 and 1.8 Hz, 1H), 8.93 (d, J = 7.8 Hz, 1H), 11.48 (bs, 1H). 13C-NMR (CDCl3, 75.5 MHz) δ 14.1 (CH3), 61.5 (CH2), 92.7 (Cq), 115.7 (Cq), 120.5 (CH), 123.0 (CH), 128.0 (CH), 128.3 (CH), 130.9 (CH), 131.4 (CH), 134.6 (CH), 140.4 (CH), 141.2 (Cq), 142.0 (Cq), 167.6 (Cq), 168.1 (Cq). IR (film) 3257, 1680 cm−1. HRMS calcd for C16H15NO3I: 396.0091. Found: 396.0091. Anal. calcd for C16H14NO3I (395.2): C, 48.63; H, 3.57; N, 3.54. Found: C, 48.61; H, 3.64; N, 3.54%. Mp 53–54 °C.N-(2-Iodophenyl)-2-nitrobenzamide 4e
To a solution of 2-iodoaniline (2.13 g, 9.72 mmol) and pyridine (2.6 mL, 32.2 mmol) in DCM (30 mL) was slowly added 2-nitrobenzoyl chloride (2 g, 9.70 mmol). The mixture was stirred overnight at rt under nitrogen. Petroleum ether was added and the resulting suspension was filtered and the solid was washed with NaHCO3 aqueous saturated solution to yield the title compound (3.54 g, 99%). 1H-NMR (CDCl3, 300 MHz) δ 6.98 (td, J = 7.5 and 1.5 Hz, 1H), 7.47 (t, J = 7.5 Hz, 1H), 7.66–7.82 (m, 3H), 7.87 (dd, J = 7.8 and 1.5 Hz, 1H), 8.19 (d, J = 8.1 Hz, 1H), 8.33 (d, J = 6.9 Hz, 1H). 13C-NMR (CDCl3, 75.5 MHz) δ 90.8 (Cq), 123.0 (CH), 124.9 (CH), 127.0 (CH), 128.3 (CH), 129.5 (CH), 131.1 (CH), 132.5 (Cq), 134.0 (CH), 137.6 (Cq), 138.9 (CH), 146.6 (Cq), 164.2 (Cq). IR (film) 1635 cm−1. HRMS calcd for C13H10O3N2I: 368.9731. Found: 368.9727. Mp 182 °C.N-Benzyl-2-nitro-N-phenylbenzamide 4f
To a solution of 2-nitrobenzoyl chloride (90%, 658 mg, 3.20 mmol) in DCM (12 mL), were added pyridine (1 mL, 12.38 mmol) and aniline (0.3 mL, 3.29 mmol), and the mixture was stirred overnight at rt under nitrogen. The mixture was basified with NaHCO3 aqueous saturated solution and extracted with DCM. The organic layer was washed with HCl 2 M, dried and filtered and the solvent was removed under reduced pressure. The residue was dissolved in dry THF (40 mL), and NaH (60%, 406 mg, 10.15 mmol) and benzyl bromide (1.6 mL, 13.4 mmol) were added and the mixture was stirred overnight at rt under nitrogen. The solvent was removed under reduced pressure, the residue was partitioned between ethyl acetate and water, and the aqueous phase was extracted with ethyl acetate. The organic extracts were dried and filtered and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (petroleum ether to petroleum ether–AcOEt 50%) to yield 4f (1.06 g, quantitative). 1H-NMR (CDCl3, 500 MHz) δ 4.96 (s, 2H), 6.73–6.76 (m, 2H), 6.86–6.89 (m, 3H), 7.06–7.17 (m, 7H), 7.23–7.27 (m, 1H), 7.73 (d, J = 8 Hz, 1H). 13C-NMR (CDCl3, 75.5 MHz) δ 52.9 (CH2), 124.3 (CH), 127.6 (CH), 127.8 (CH), 128.2 (CH), 128.5 (CH), 128.9 (CH), 129.1 (CH), 129.29 (CH), 129.34 (CH), 133.26 (Cq), 133.34 (CH), 136.6 (Cq), 141.1 (Cq), 155.7 (Cq), 167.1 (Cq). IR (film) 1651 cm−1. HRMS calcd for C20H16N2O3Na: 355.1053. Found: 355.1051. Mp 97–99 °C.2-Amino-N-benzyl-N-phenylbenzamide 4g
To a solution of 4f (1 g, 3.00 mmol) in DMF (30 mL) was added SnCl2·H2O (7.7 g, 34.1 mmol). The mixture was stirred at rt for 24 h and was basified with NaHCO3 aqueous saturated solution and extracted with DCM. The organic extracts were washed with brine, dried and filtered and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (petroleum ethereum ether to AcOEt) to yield 4g (783 mg, 86%). 1H-NMR (CDCl3, 500 MHz) δ 4.52 (bs, 2H), 5.02 (s, 2H), 6.21 (td, J = 7.5 and 1 Hz, 1H), 6.53 (dd, J = 8.1 and 1 Hz, 1H), 6.66 (dd, J = 7.8 and 1.5 Hz, 1H), 6.83–6.87 (m, 3H), 6.93–6.97 (m, 1H), 6.98–7.03 (m, 2H), 7.10–7.22 (m, 6H). 13C-NMR (CDCl3, 125 MHz) δ 53.3 (CH2), 116.6 (CH), 116.9 (CH), 119.9 (Cq), 126.5 (CH), 126.9 (2CH), 127.3 (CH), 128.1 (2CH), 128.5 (2CH), 128.9 (2CH), 129.6 (CH), 130.5 (CH), 137.5 (Cq), 143.6 (Cq), 146.4 (Cq), 170.9 (Cq). IR (film) 3359 and 1619 cm−1. HRMS calcd for C20H19N2O: 303.1492. Found: 303.1501.2-Iodo-N-benzyl-N-(2-iodophenyl)benzamide 3a
To a suspension of NaH (60%, 37 mg, 0.92 mmol) in THF (5 mL) were added 2-iodo-N-(2-iodophenyl)benzamide 4a (110 mg, 0.24 mmol) and benzyl bromide (110 µL, 0.92 mmol). The mixture was stirred overnight at rt under nitrogen. The mixture was diluted with ethyl acetate and washed with water. The organic layer was dried and filtered and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (petroleum ether to petroleum ether–AcOEt 20%) to yield 3a (110 mg, 91%). 1H-NMR (CDCl3, 300 MHz) major conformer δ 4.22 (d, J = 14.1 Hz, 1H), 6.00 (d, J = 14.1 Hz, 1H), 6.83–6.76 (m, 1H), 6.86 (dd, J = 5.7 and 1.8 Hz, 1H), 6.91 (dd, J = 6.9 and 1.5 Hz, 1H), 6.93 (dd, J = 6.9 and 1.5 Hz, 1H), 7.08 (ddd, J = 7.8, 7.5 and 1.2 Hz, 1H), 7.26–7.29 (m, 3H), 7.37–7.40 (m, 3H), 7.66 (dd, J = 8.1 and 1.2 Hz, 1H), 7.79 (dd, J = 7.8 and 1.2 Hz, 1H). Minor conformer δ 4.60 (d, J = 14.1 Hz, 1H), 4.73 (d, J = 14.1 Hz, 1H), 6.76–8.01 (m, 13H masked by the major conformer). Ratio 10 : 1. 13C-NMR (CDCl3, 75.5 MHz) δ 51.3 (CH2), 94.0 (Cq), 99.3 (Cq), 126.4 (CH), 127.3 (CH), 127.7 (CH), 128.1 (CH), 128.6 (CH), 129.5 (CH), 130.0 (CH), 131.8 (CH), 136.0 (Cq), 139.2 (CH), 139.8 (CH), 141.4 (Cq), 142.8 (Cq), 169.5 (Cq). IR (film) 1655 cm−1. HRMS calcd for C20H16NOI2: 539.9316. Found: 539.9321. Mp 118–119 °C.2-Iodo-N-(2-iodobenzyl)-N-(2-iodophenyl)benzamide 3b
To a suspension of NaH (60%, 40 mg, 1 mmol) in THF (5 mL) were added 2-iodo-N-(2-iodophenyl)benzamide 4a (104 mg, 0.23 mmol) and 2-iodobenzyl bromide (276 mg, 0.93 mmol). The mixture was stirred overnight at rt under nitrogen. The mixture was diluted with ethyl acetate and washed with water. The organic layer was dried and filtered and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (petroleum ether to petroleum ether–AcOEt 25%) to yield 3b (140 mg, 88%). 1H-NMR (CDCl3, 300 MHz) conformer A δ 4.62 (d, J = 14.5 Hz, 1H), 6.00 (d, J = 14.5 Hz, 1H), 6.80 (dd, J = 7.5 and 1.5 Hz, 1H), 6.82 (dd, J = 7.5 and 1.5 Hz, 1H), 6.85 (dd, J = 7.5 and 1.5 Hz, 1H), 6.92 (dd, J = 7.5 and 1.5 Hz, 1H), 6.95 (dd, J = 7.5 and 1.5 Hz, 1H), 7.09 (ddd, J = 7.8, 7.5 and 1.2 Hz, 1H), 7.37 (ddd, J = 7.8, 7.5 and 1.5 Hz, 1H), 7.42 (dd, J = 7.8 and 1.5 Hz, 1H), 7.66 (dd, J = 7.8 and 1.5 Hz, 1H), 7.67 (dd, J = 7.8 and 1.5 Hz, 1H), 7.77 (dd, J = 7.8 and 1.5 Hz, 1H), 8.07 (dd, J = 7.8 and 1.5 Hz, 1H). Conformer B δ 4.81 (d, J = 14.5 Hz, 1H), 5.97 (d, J = 14.5 Hz, 1H), 6.78–8.09 (m, 12H masked by the major conformer). Conformer C δ 4.89 (d, J = 14.5 Hz, 1H), 4.99 (d, J = 14.5 Hz, 1H), 6.78–8.09 (m, 12H masked by the major conformer). 13C-NMR (CDCl3, 75.5 MHz) δ 54.4 (CH2), 93.9 (Cq), 100.5 (Cq), 100.8 (Cq), 126.7 (CH), 127.4 (CH), 128.5 (CH), 128.8 (CH), 129.5 (CH), 129.7 (CH), 130.1 (CH), 131.6 (CH), 131.7 (CH), 139.1 (CH), 139.26 (CH), 139.27 (Cq), 140.0 (CH), 141.0 (Cq), 142.2 (Cq), 170.0 (Cq). Ratio 16 : 1.2 : 1. IR (film) 1650 cm−1. HRMS calcd for C20H14NOI3Na: 687.8102. Found: 687.8089. Mp 182–184 °C.2-Iodo-N-ethyl-N-(2-iodophenyl)benzamide 3c
To a suspension of NaH (60%, 70 mg, 1.75 mmol) in THF (5 mL) was added 2-iodo-N-(2-iodophenyl)benzamide 4a (199 mg, 0.40 mmol) and iodoethane (100 µL, 1.24 mmol). The mixture was stirred at rt under nitrogen overnight. The mixture was diluted with ethyl acetate and washed with water. The organic layer was dried and filtered and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (petroleum ether–AcOEt 10%) to yield 3c (196 mg, 92%). 1H-NMR (CDCl3, 300 MHz) major conformer δ 1.29 (dd, J = 7.2 and 6.9 Hz, 3H), 3.22 (dq, J = 14 and 6.9 Hz, 1H), 4.63 (dq, J = 14 and 7.2 Hz, 1H), 6.81 (dd, J = 7.5 and 1.5 Hz, 1H), 6.89 (dd, J = 7.5 and 1.5 Hz, 1H), 7.07 (td, J = 7.5 and 1.2 Hz, 1H), 7.16 (td, J = 7.5 and 1.5 Hz, 1H), 7.29 (dd, J = 7.5 and 1.5 Hz, 1H), 7.41 (dd, J = 7.8 and 1.5 Hz, 1H), 7.69 (dd, J = 7.8 and 1.2 Hz, 1H), 7.81 (dd, J = 7.8 and 1.5 Hz, 1H). Minor conformer δ 1.10 (dd, J = 6.9 and 7.2 Hz, 3H), 3.53 (m, 2H), 6.80–7.50 (m, 6H masked by the major conformer), 7.89 (dd, J = 7.8 and 1.2 Hz, 1H), 7.96 (d, J = 7.8 Hz, 1H). Ratio 4.3 : 1. 13C-NMR (CDCl3, 75.5 MHz) δ 12.2 (CH3), 43.3 (CH2), 94.1 (Cq), 99.7 (Cq), 126.2 (CH), 127.4 (CH), 128.6 (CH), 129.4 (CH), 129.9 (CH), 130.9 (CH), 139.0 (CH), 139.9 (CH), 141.6 (Cq), 143.1 (Cq), 169.2 (Cq). IR (film) 1651 cm−1. HRMS calcd for C15H14NOI2: 477.9159. Found: 477.9163. Mp 139–140 °C.2-Iodo-N-(2-iodophenyl)-N-isopropylbenzamide 3d
To a solution of 2-iodo-N-isopropylaniline20 (174 mg, 0.67 mmol) and pyridine (0.27 mL, 3.68 mmol) in dry DCM (6 mL) was added 2-iodobenzoyl chloride (268 mg, 1.00 mmol). The mixture was stirred overnight at rt under nitrogen. The mixture was diluted with DCM and washed with saturated aqueous NaHCO3. The organic layer was dried and filtered and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (petroleum ether to petroleum ether–AcOEt 10%) to yield 3d (246 mg, 75%). 1H-NMR (CDCl3, 300 MHz) major conformer δ 1.29 (d, J = 6.6 Hz, 3H), 1.66 (d, J = 6.6 Hz, 3H), 4.61 (h, J = 6.6 Hz, 1H), 6.81 (dd, J = 7.5 and 1.5 Hz, 1H), 6.90 (dd, J = 7.5 and 1.5 Hz, 1H), 7.11 (td, J = 7.5 and 1.2 Hz, 1H), 7.21 (td, J = 7.5 and 1.5 Hz, 1H), 7.60 (dd, J = 7.8 and 1.5 Hz, 1H), 7.62 (dd, J = 7.8 and 1.5 Hz, 1H), 7.65 (dd, J = 7.8 and 1.5 Hz, 1H), 7.79 (dd, J = 7.8 and 1.5 Hz, 1H). Minor conformer δ 1.16 (d, J = 6.7 Hz, 3H), 1.34 (d, J = 6.7 Hz, 3H), 4.13 (h, J = 6.7 Hz, 1H), 6.81–7.72 (m, 6H masked by the major conformer), 7.91 (d, J = 7.8 Hz, 1H), 8.00 (d, J = 7.8 Hz, 1H). Ratio 2.5 : 1. 13C-NMR (CDCl3, 75.5 MHz) major conformer δ 19.4 (CH3), 21.6 (CH3), 51.5 (CH), 93.9 (Cq), 102.8 (Cq), 126.6 (CH), 127.1 (CH), 128.6 (CH), 129.4 (CH), 129.7 (CH), 130.9 (CH), 139.0 (CH), 140.0 (CH), 142.1 (Cq), 142.8 (Cq), 169.7 (Cq). Minor conformer δ 20.8 (CH3), 23.7 (CH3), 52.8 (CH), 92.9 (Cq), 102.3 (Cq), 126.7 (CH), 128.0 (CH), 128.9 (CH), 129.4 (CH), 130.1 (CH), 130.8 (CH), 139.4 (CH), 140.1 (CH), 169.4 (Cq). IR (film) 1649 cm−1. HRMS calcd for C16H16NOI2: 491.9316. Found: 491.9322. Mp 161–162 °C.N-Benzyl-N-(2-iodophenyl)-1-naphthamide 3e
To a suspension of NaH (60%, 94 mg, 2.35 mmol) in THF (10 mL) was added N-(2-iodophenyl)-1-naphthamide 4b (257 mg, 0.69 mmol) and benzyl bromide (350 µL, 2.93 mmol). The mixture was stirred overnight at rt under nitrogen. The mixture was diluted with ethyl acetate and washed with water. The organic layer was dried and filtered and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (petroleum ether to petroleum ether–AcOEt 20%) to yield 3e (296 mg, 93%). 1H-NMR (CDCl3, 300 MHz) major conformer δ 4.32 (d, J = 14.1 Hz, 1H), 6.12 (d, J = 14.1 Hz, 1H), 6.37 (m, 1H), 6.66 (m, 1H), 7.16 (d, J = 7.2 Hz, 1H), 7.19 (d, J = 7.2 Hz, 1H), 7.27–7.53 (m, 7H), 7.63 (d, J = 8.4 Hz, 1H), 7.70–7.73 (m, 1H), 7.74 (d, J = 7.2, 1H), 8.09 (d, J = 8.4 Hz, 1H). Minor conformer δ 4.54 (bd, J = 14.1 Hz, 1H), 4.82 (m, 1H), 4.40–7.99 (m, 16H masked by the major conformer). Ratio 7 : 1. 13C-NMR (CDCl3, 75.5 MHz) δ 51.6 (CH2), 99.4 (Cq), 123.7 (CH), 124.2 (CH), 125.2 (CH), 125.9 (CH), 126.6 (CH), 127.7 (CH), 128.1 (2CH), 128.4 (2CH), 129.0 (CH), 129.1 (CH), 129.6 (2CH), 130.2 (Cq), 131.3 (CH), 133.1 (Cq), 133.6 (Cq), 136.8 (Cq), 139.8 (CH), 143.4 (Cq), 170.0 (Cq). IR (film) 1649 cm−1. HRMS calcd for C24H19NOI: 464.0506. Found: 464.0507. Anal. calcd for C24H18NOI (463.3)·1/4 H2O: C, 61.62; H, 3.99; N, 2.99. Found: C, 61.55; H, 3.98; N, 2.94%. Mp 182–184 °C.Ethyl 2-(N-benzyl-1-naphthamido)benzoate 3f
To a suspension of NaH (60%, 111 mg, 2.77 mmol) in THF (10 mL) were added ethyl 2-(1-naphthamido)benzoate 4c (251 mg, 0.79 mmol) and benzyl bromide (400 µL, 3.34 mmol). The mixture was stirred overnight at rt under nitrogen. The mixture was diluted with ethyl acetate and washed with water. The organic layer was dried and filtered and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (petroleum ether to petroleum ether–AcOEt 30%) to yield 3f (193 mg, 60%). 1H-NMR (CDCl3, 300 MHz) major conformer δ 1.45 (t, J = 6.9 Hz, 3H), 4.28 (d, J = 14.4 Hz, 1H), 4.36–4.49 (m, 2H), 6.21 (d, J = 14.4 Hz, 1H), 6.59 (d, J = 7.8 Hz, 1H), 6.94 (td, J = 7.5 and 1.5 Hz, 1H), 7.06 (td, J = 7.5 and 1.2 Hz, 1H), 7.15 (d, J = 8.1 Hz, 1H), 7.17 (d, J = 8.1 Hz, 1H), 7.30–7.48 (m, 5H), 7.53 (dd, J = 8.4 and 1.5 Hz, 1H), 7.55 (dd, J = 8.4 and 1.5 Hz, 1H), 7.64 (d, J = 8.1 Hz, 1H), 7.73 (d, J = 8.4 Hz, 1H), 7.76 (dd, J = 7.5 and 1.5 Hz, 1H), 8.17 (d, J = 8.1 Hz, 1H). Minor conformer δ 1.48 (t, J = 6.9 Hz, 3H), 4.35–4.49 (m, 4H masked by the major conformer), 6.88–7.98 (m, 15H masked by the major conformer), 8.11 (dd, J = 7.5 and 1.5 Hz, 1H). Ratio 5 : 1. 13C-NMR (CDCl3, 75.5 MHz) δ 14.1 (CH3), 53.1 (CH2), 61.4 (CH2), 124.1 (CH), 124.6 (CH), 125.3 (CH), 125.8 (CH), 126.4 (CH), 127.46 (CH), 127.50 (CH), 128.0 (CH), 128.2 (Cq), 128.4 (2CH), 128.9 (CH), 129.3 (2CH), 130.2 (Cq), 130.9 (CH), 131.3 (CH), 131.9 (CH), 133.1 (Cq), 133.8 (Cq), 137.4 (Cq), 141.7 (Cq), 164.5 (Cq), 169.5 (Cq). IR (film) 1718 and 1648 cm−1. HRMS calcd for C27H24NO3: 410.1751. Found: 410.1750. Anal. calcd for C27H23NO3 (409.5)·1/4 H2O: C, 78.34; H, 5.72; N, 3.38. Found: C, 78.20; H, 5.81; N, 3.28%.2-(N-Benzyl-1-naphthamido)benzoic acid 3g
To a solution of 3f (516 mg, 0.99 mmol) in EtOH (10 mL) was added NaOH 40% aqueous solution (40 mL) and the mixture was stirred at reflux temperature for 3 h. The mixture was acidified with HCl 6 N and extracted with ethyl acetate. The organic extracts were dried and filtered and the solvent was removed under reduced pressure to yield 3g (468 mg, 97%). 1H-NMR (CDCl3, 300 MHz) major conformer δ 4.33 (d, J = 14.4 Hz, 1H), 6.19 (d, J = 14.4 Hz, 1H), 6.61 (bd, J = 6.9 Hz, 1H), 6.98–7.04 (m, 1H), 7.10–7.21 (m, 3H), 7.30–7.59 (m, 7H), 7.67 (d, J = 8.1 Hz, 1H), 7.75 (d, J = 7.8 Hz, 1H), 7.91 (d, J = 7.5 Hz, 1H), 8.19 (d, J = 7.8 Hz, 1H). Minor conformer δ 4.60–5.00 (m, 4H), 6.87–6.89 (m, 1H), 6.99–7.76 (m, 13H masked by the major conformer), 7.98 (d, J = 7.5 Hz, 1H), 8.23 (d, J = 7.2 Hz, 1H). Ratio 3.25 : 1. 13C-NMR (CDCl3, 75.5 MHz) δ 53.4 (CH2), 124.4 (CH), 125.3 (CH), 126.0 (CH), 126.7 (CH), 126.9 (CH), 127.6 (CH), 127.8 (CH), 128.2 (CH), 128.3 (Cq), 128.5 (2CH), 129.2 (CH), 129.5 (2CH), 130.2 (Cq), 131.2 (CH), 132.3 (CH), 133.0 (CH), 133.2 (Cq), 133.7 (Cq), 137.2 (Cq), 142.4 (Cq), 169.7 (Cq), 170.1 (Cq). IR (film) 3060, 1718 and 1594 cm−1. HRMS calcd for C25H19NO3Na: 404.1257. Found: 404.1260.Ethyl 2-(N-benzyl-2-iodobenzamido)benzoate 3h
To a solution of 4d (1 g, 2.53 mmol), in dry THF (40 mL), were added NaH (60%, 320 mg, 8 mmol) and benzyl bromide (1.25 mL, 10.4 mmol) and the mixture was stirred overnight at rt under nitrogen. The mixture was diluted with ethyl acetate and washed with water. The organic layer was dried and filtered and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (petroleum ether to petroleum ether–AcOEt 40%) to yield 3h (834 mg, 68%). 1H-NMR (CDCl3, 300 MHz) major conformer δ 1.53 (t, J = 7.1 Hz, 3H), 4.09 (d, J = 14.3 Hz, 1H), 4.52 (q, J = 7.1 Hz, 2H), 6.03 (d, J = 14.3 Hz, 1H), 6.83 (ddd, J = 8, 7.5 and 1.8 Hz, 1H), 6.96 (dd, J = 7.5 and 1.8 Hz, 1H), 7.06 (td, J = 7.5 and 1 Hz, 1H), 7.13–7.22 (m, 3H), 7.28–7.32 (m, 3H), 7.39–7.42 (m, 2H), 7.68 (dd, J = 8 and 1 Hz, 1H), 7.87 (dd, J = 7.5 and 1.8 Hz, 1H). Minor conformer δ 1.41 (t, J = 7.1 Hz, 3H), 4.30–4.80 (m, 4H masked by the major conformer), 6.80–7.44 (m, 8H masked by the major conformer), 7.50 (dd, J = 7.8 and 1.8 Hz, 1H), 7.55 (dd, J = 7.5 and 1 Hz, 1H), 7.78 (dd, J = 7.6 and 1 Hz, 1H), 7.94 (dd, J = 8 and 1 Hz, 1H), 8.02 (dd, J = 7.8 and 1.5 Hz, 1H). Ratio 5.3 : 1. 13C-NMR (CDCl3, 75.5 MHz) δ 14.3 (CH3), 52.9 (CH2), 61.7 (CH2), 94.0 (Cq), 127.1 (CH), 127.3 (CH), 127.5 (CH), 128.0 (CH), 128.2 (2CH), 129.6 (2CH), 129.7 (CH), 131.5 (2CH), 132.4 (CH), 136.5 (Cq), 139.1 (CH), 140.9 (Cq), 142.0 (Cq), 165.7 (Cq), 169.1 (Cq). IR (film) 1716 and 1652 cm−1. HRMS calcd for C23H21NO3I: 486.0561. Found: 486.0570. Anal. calcd for C23H20NO3I (485.3): C, 56.92; H, 4.15; N, 2.89. Found: C, 57.36; H, 4.28; N, 2.79%. Mp 102–104 °C.2-(N-Benzyl-2-iodobenzamido)benzoic acid 3i
To a solution of 3h (405 mg, 0.83 mmol) in EtOH (10 mL) was added NaOH 40% aqueous solution (30 mL) and the mixture was stirred at reflux temperature for 3 h. The mixture was acidified with HCl 6 N and extracted with DCM. The organic extracts were dried and filtered and the solvent was removed under reduced pressure to yield 3i (382 mg, quantitative). 1H-NMR (CDCl3, 300 MHz) major conformer δ 4.90 (d, J = 14.4 Hz, 1H), 6.10 (d, J = 14.4 Hz, 1H), 6.84 (ddd, J = 7.8, 7.5 and 1.5 Hz, 1H), 7.00–7.12 (m, 2H), 7.17–7.35 (m, 6H), 7.42–7.45 (m, 2H), 7.70 (d, J = 7.8 Hz, 1H), 8.01 (dd, J = 7.5 and 1.8 Hz, 1H), 10.37 (br s, 1H). Minor conformer δ 4.67–4.73 (m, 1H), 4.86–4.91 (m, 1H), 7.00–7.75 (m, 11H masked by the major conformer), 7.97 (d, J = 7.8 Hz, 1H), 8.19 (dd, J = 7.8 and 1.2 Hz, 1H). Ratio 3.5 : 1. 13C-NMR (CDCl3, 75.5 MHz) δ 53.2 (CH2), 93.9 (Cq), 127.1 (Cq), 127.3 (CH), 127.5 (CH), 127.6 (CH), 128.26 (2CH), 128.30 (CH), 129.7 (2CH), 129.9 (CH), 131.6 (CH), 132.4 (CH), 133.3 (CH), 136.3 (Cq), 139.1 (CH), 141.4 (Cq), 141.6 (Cq), 169.6 (Cq), 169.7 (Cq). IR (film) 3031, 1720 and 1596 cm−1. HRMS calcd for C21H15NO3I: 456.0102. Found: 456.0096.N-Benzyl-N-(2-iodophenyl)-2-nitrobenzamide 3j
To a solution of 4e (1.2 g, 3.26 mmol), in dry THF (40 mL), were added NaH (60%, 460 mg, 11.5 mmol) and benzyl bromide (1.6 mL, 13.4 mmol) and the mixture was stirred overnight at rt under nitrogen. The solvent was evaporated under reduced pressure. The residue was partitioned between ethyl acetate and water, and the aqueous phase was extracted with ethyl acetate. The organic extracts were dried and filtered and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (petroleum ether to petroleum ether–AcOEt 50%) to yield 3j (1.43 g, 96%). 1H-NMR (CDCl3, 300 MHz) major conformer δ 4.31 (d, J = 14.1 Hz, 1H), 6.01 (d, J = 14.1 Hz, 1H), 6.72 (dd, J = 7.8 and 1.8 Hz, 1H), 6.83 (td, J = 7.5 and 1.8 Hz, 1H), 6.93 (td, J = 7.5 and 1.5 Hz, 1H), 7.30–7.42 (m, 6H), 7.51 (td, J = 7.5 and 1.5 Hz, 1H), 7.75 (dd, J = 7.8 and 1.5 Hz, 1H), 7.80 (dd, J = 7.8 and 1.5 Hz, 1H), 7.97 (dd, J = 8.1 and 1.5 Hz, 1H). Minor conformer δ 4.71 (s, 2H), 6.70–7.81 (m, 10H masked by the major conformer), 7.90 (dd, J = 7.8 and 1.5 Hz, 1H), 7.91 (dd, J = 7.8 and 1.5 Hz, 1H), 8.35 (dd, J = 8.1 and 1.5 Hz, 1H). Ratio 12 : 1. 13C-NMR (CDCl3, 75.5 MHz) δ 51.4 (CH2), 99.5 (Cq), 124.2 (CH), 127.8 (CH), 127.9 (CH), 128.4 (2CH), 128.9 (CH), 129.7 (CH), 129.89 (CH), 129.92 (2CH), 131.2 (CH), 132.7 (Cq), 133.6 (CH), 135.5 (Cq), 140.0 (CH), 142.3 (Cq), 145.4 (Cq), 166.5 (Cq). IR (film) 1653, 1527 and 1346 cm−1. HRMS calcd for C20H15N2O3INa: 481.0020. Found: 481.0016. Mp 158–160 °C.N-Ethyl-N-(2-iodophenyl)-2-nitrobenzamide 3k
To a solution of 4e (503 mg, 1.37 mmol), in dry THF (10 mL), were added NaH (60%, 257 mg, 6.4 mmol) and ethyl iodide (1 mL, 12.4 mmol) and the mixture was stirred overnight at rt under nitrogen. The solvent was evaporated under reduced pressure. The residue was partitioned between ethyl acetate and water, and the aqueous phase was extracted with ethyl acetate. The organic extracts were dried and filtered and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (petroleum ether to petroleum ether–AcOEt 50%) to yield 3k (458 mg, 85%). 1H-NMR (CDCl3, 300 MHz) major conformer δ 1.34 (t, J = 7.2 Hz, 3H), 3.28 (dq, J = 14 and 7.2 Hz, 1H), 4.60 (dq, J = 14 and 7.2 Hz, 1H), 6.91 (td, J = 7.5 and 1.5 Hz, 1H), 7.16 (td, J = 7.5 and 1.5 Hz, 1H), 7.30 (dd, J = 8.1 and 1.5 Hz, 1H), 7.37 (ddd, J = 8.1, 7.5 and 1.5 Hz, 1H), 7.47 (td, J = 7.5 and 1.5 Hz, 1H), 7.61 (dd, J = 7.5 and 1.5 Hz, 1H), 7.82 (dd, J = 7.8 and 1.5 Hz, 1H), 7.98 (dd, J = 7.5 and 1.5 Hz, 1H). Minor conformer δ 1.06 (t, J = 7.2 Hz, 3H), 3.55 (q, J = 7.2 Hz, 2H), 6.8–8.0 (m, 7H masked by the major conformer), 8.29 (dd, J = 8.1 and 1.5 Hz, 1H). Ratio 6.4 : 1. 13C-NMR (CDCl3, 75.5 MHz) major conformer δ 11.6 (CH3), 43.6 (CH2), 100.1 (Cq), 124.2 (CH), 127.4 (CH), 129.2 (CH), 129.6 (CH), 129.8 (CH), 130.2 (CH), 132.9 (Cq), 133.6 (CH), 140.1 (CH), 143.2 (Cq), 166.1 (Cq). Minor conformer representative signals only, δ 13.3 (CH3), 46.4 (CH2), 124.8 (CH), 128.1 (CH), 129.7 (CH), 130.1 (CH), 134.5 (CH), 139.7 (CH), 145.9 (Cq). IR (film) 1655 cm−1. HRMS calcd for C15H14N2O3I: 397.0044. Found: 397.0043.N-Methyl-N-(2-iodophenyl)-2-nitrobenzamide 3l
To a solution of 4e (501 mg, 1.36 mmol), in dry THF (10 mL), were added NaH (60%, 235 mg, 5.9 mmol) and methyl iodide (0.9 mL, 14.5 mmol) and the mixture was stirred overnight at rt under nitrogen. The solvent was evaporated under reduced pressure. The residue was partitioned between ethyl acetate and water, and the aqueous phase was extracted with ethyl acetate. The organic extracts were dried and filtered and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (petroleum ether to AcOEt) to yield 3l (407 mg, 78%). 1H-NMR (CDCl3, 300 MHz) major conformer δ 3.43 (s, 3H), 6.87 (td, J = 7.5 and 1.5 Hz, 1H), 7.14 (td, J = 7.5 and 1.5 Hz, 1H), 7.32 (dd, J = 7.5 and 1.5 Hz, 1H), 7.34 (ddd, J = 8.1, 7.5 and 1.5 Hz, 1H), 7.46 (td, J = 7.5 and 1.2 Hz, 1H), 7.62 (dd, J = 7.5 and 1.5 Hz, 1H), 7.76 (dd, J = 7.5 and 1.5 Hz, 1H), 7.95 (dd, J = 8.1 and 1.2 Hz, 1H). Minor conformer δ 3.09 (s, 3H), 7.10 (ddd, J = 8.1, 7.5 and 1.5 Hz, 1H), 7.50 (td, J = 7.5 and 1.5 Hz, 1H), 7.59–7.84 (m, 4H masked by the major conformer), 7.94 (dd, J = 8.1 and 1.2 Hz, 1H), 8.27 (dd, J = 8.1 and 1.2 Hz, 1H). Ratio 3.6 : 1. 13C-NMR (CDCl3, 75.5 MHz) major conformer δ 36.7 (CH3), 99.1 (Cq), 124.2 (CH), 127.9 (CH), 128.9 (CH), 129.71 (CH), 129.75 (CH), 129.96 (CH), 132.7 (Cq), 133.6 (CH), 140.1 (CH), 145.1 (Cq), 167.1 (Cq). Minor conformer representative signals only, δ 39.1 (CH3), 124.7 (CH), 128.2 (CH), 128.7 (CH), 129.8 (CH), 130.00 (CH), 130.1 (CH), 132.7 (CH), 134.8 (CH), 145.6 (Cq). IR (film) 1654 cm−1. HRMS calcd for C14H12N2O3I: 382.9887. Found: 382.9883.2-Amino-N-benzyl-N-(2-iodophenyl)benzamide 3m
To a solution of 3j (400 mg, 0.87 mmol) in DMF (9 mL) was added SnCl2·H2O (2.02g, 8.94 mmol). The mixture was stirred at rt for 24 h and it was basified with NaHCO3 aqueous saturated solution and extracted with DCM. The organic extracts were washed with brine, dried and filtered and the solvent was removed under reduced pressure to yield the title compound 3m (374 mg, quantitative). 1H-NMR (CDCl3, 300 MHz) δ 3.96 (bs, 2H), 4.39 (d, J = 14.4 Hz, 1H), 5.77 (d, J = 14.4 Hz, 1H), 6.34 (bs, 1H), 6.62 (d, J = 7.8 Hz, 1H), 6.71 (d, J = 8.1 Hz, 1H), 6.82–7.03 (m, 4H), 7.20–7.37 (m, 5H), 7.82 (d, J = 7.8 Hz, 1H). 13C-NMR (CDCl3, 125 MHz) δ 51.8 (CH2), 99.2 (Cq), 116.0 (CH), 116.2 (CH), 118.8 (Cq), 127.3 (CH), 128.1 (CH), 128.2 (2CH), 128.5 (2CH), 128.6 (CH), 129.0 (CH), 130.5 (CH), 131.1 (CH), 136.4 (Cq), 139.7 (CH), 144.4 (Cq), 146.7 (Cq), 170.5 (Cq). IR (film) 3364 and 1634 cm−1. HRMS calcd for C20H18N2OI: 429.0458. Found: 429.0462.2-Amino-N-ethyl-N-(2-iodophenyl)benzamide 3n
To a solution of 3k (458 mg, 1.16 mmol) in DMF (10 mL) was added SnCl2·H2O (2.77 g, 12.3 mmol). The mixture was stirred at rt for 24 h and it was basified with NaHCO3 aqueous saturated solution and extracted with DCM. The organic extracts were washed with brine, dried and filtered and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (petroleum ether to AcOEt) to yield 3n (417 mg, 98%). 1H-NMR (CDCl3, 300 MHz) δ 1.21 (t, J = 7.2 Hz, 3H), 3.44–3.55 (m, 1H), 4.29 (bs, 1H), 4.65 (bs, 2H), 6.31 (bs, 1H), 6.58 (d, J = 7.5 Hz, 1H), 6.80–7.20 (m, 5H), 7.84 (d, J = 7.2 Hz, 1H). 13C-NMR (CDCl3, 75.5 MHz) δ 12.2 (CH3), 44.5 (CH2), 99.9 (Cq), 116.7 (CH), 116.8 (CH), 119.9 (Cq), 128.6 (CH), 129.0 (CH), 129.2 (CH), 130.9 (CH), 131.1 (CH), 140.3 (CH), 147.0 (Cq), 170.9 (Cq). IR (film) 3360 and 1620 cm−1. HRMS calcd for C15H16N2OI: 367.0302. Found: 367.0302.2-Amino-N-(2-iodophenyl)-N-methylbenzamide 3o
To a solution of 3l (595 mg, 1.56 mmol) in DMF (10 mL) was added SnCl2·H2O (3.7 g, 16.40 mmol). The mixture was stirred at rt for 24 h and it was basified with NaHCO3 aqueous saturated solution and extracted with DCM. The organic extracts were washed with brine, dried and filtered and the solvent was removed under reduced pressure to yield 3o (548 mg, quantitative). 1H-NMR (CDCl3, 300 MHz) δ 3.36 (s, 3H), 4.73 (bs, 2H), 6.39 (bs, 1H), 6.69 (d, J = 8.4 Hz, 1H), 6.83–7.21 (m, 5H), 7.83 (d, J = 7.8 Hz, 1H). 13C-NMR (CDCl3, 75.5 MHz) δ 37.8, (CH3), 98.8 (Cq), 116.8 (CH), 119.1 (Cq), 129.1 (CH), 129.7 (CH), 131.1 (CH), 140.3 (CH), 147.2 (Cq), 171.4 (Cq). IR (film) 3358 and 1620 cm−1.N-Benzyl-N-(2-iodophenyl)-2-(2-iodobenzamido)benzamide 5a
To a solution of 3m (389 mg, 0.91 mmol) and pyridine (0.5 mL, 6.18 mmol) in DCM (5 mL) was added 2-iodobenzoyl chloride (97%, 250 mg, 0.91 mmol). The mixture was stirred at rt for 24 h and it was basified with NaHCO3 aqueous saturated solution and extracted with DCM. The organic extracts were dried and filtered and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (DCM to DCM–MeOH 0.5%) to yield 5a (436 mg, 73%). 1H-NMR (CDCl3, 300 MHz) major conformer δ 4.35 (d, J = 14.4 Hz, 1H), 5.72 (d, J = 14.4 Hz, 1H), 6.75–6.72 (m, 2H), 6.91 (ddd, J = 7.8, 7.5 and 1.5 Hz, 1H), 7.03 (dd, J = 7.8 and 1.5 Hz, 1H), 7.13 (ddd, J = 7.8, 7.5 and 1.5 Hz, 1H), 7.19–7.40 (m, 6H), 7.49–7.51 (m, 2H), 7.82 (dd, J = 8.1 and 1.5 Hz, 1H), 7.99 (d, J = 7.8 Hz, 1H), 8.38 (d, J = 8.1 Hz, 1H), 9.73 (s, 1H). Minor conformer δ 4.57–4.64 (m, 1H), 5.21–5.27 (m, 1H), other signals masked by the major conformer. Ratio 12 : 1. 13C-NMR (CDCl3, 75.5 MHz) δ 53.7 (CH2), 92.8 (Cq), 99.8 (Cq), 122.5 (CH), 122.8 (CH), 124.4 (Cq), 127.76 (CH), 127.79 (CH), 128.2 (CH), 128.3 (CH), 128.4 (2CH), 128.9 (CH), 129.3 (2CH), 130.8 (CH), 131.3 (CH), 131.4 (CH), 136.1 (Cq), 137.1 (Cq), 140.1 (CH), 140.3 (CH), 141.7 (Cq), 144.1 (Cq), 167.1 (Cq), 169.5 (Cq). IR (film) 3338, 1683 and 1632 cm−1. HRMS calcd for C27H20N2O2I2Na: 680.9506. Found: 680.9507. Mp 112–114 °C.N-Benzyl-N-(2-iodophenyl)-2-(2-iodo-N-methylbenzamido)benzamide 5b
To a solution of 5a (95 mg, 0.14 mmol), in dry THF (5 mL), were added NaH (60%, 30 mg, 0.75 mmol) and methyl iodide (0.1 mL, 1.60 mmol) and the mixture was stirred overnight at rt under nitrogen. The residue was partitioned between DCM and water, and the aqueous phase was extracted with DCM. The organic extracts were dried and filtered and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (petroleum ether to petroleum ether–AcOEt 30%) to yield 5b (90 mg, 93%). 1H-NMR (CDCl3, 300 MHz) conformer A δ 3.24 (br s, 3H), 4.55 (d, J = 14.5 Hz, 1H), 6.72 (d, J = 14.5 Hz, 1H), 6.81–7.94 (m, 17H). Conformer B δ 4.90 (d, J = 15 Hz, 1H), 5.20 (d, J = 15 Hz, 1H), other signals masked by the major conformer. Conformer C δ 4.32 (d, J = 14 Hz, 1H), 6.98 (d, J = 14 Hz, 1H), other signals masked by the major conformer. Ratio 6.8 : 2 : 1. 13C-NMR (CDCl3, 75.5 MHz) δ 41.5 (CH3), 53.1 (CH2), 92.0 (Cq), 98.2 (Cq), 126.9 (CH), 127.3 (2CH), 128.32 (2CH), 128.41 (CH), 128.44 (CH), 128.5 (CH), 129.0 (CH), 129.1 (CH), 129.5 (CH), 130.1 (CH), 130.9 (CH), 133.6 (Cq), 133.9 (CH), 136.8 (Cq), 138.8 (CH), 139.8 (CH), 141.8 (Cq), 142.3 (Cq), 144.5 (Cq), 167.4 (Cq), 170.7 (Cq). IR (film) 1652 cm−1. HRMS calcd for C28H22N2O2I2Na: 694.9663. Found: 694.9672. Mp 156–158 °C. HPLC 5.49 min.N-Benzyl-N-(2-iodophenyl)-2-(N-benzyl-2-iodobenzamido)benzamide 5c
To a solution of 5a (200 mg, 0.30 mmol), in dry THF (5 mL), was added NaH (60%, 54 mg, 1.35 mmol) and benzyl bromide (0.2 mL, 1.67 mmol) and the mixture was stirred overnight at rt under nitrogen. The mixture was partitioned between ethyl acetate and water, and the aqueous phase was extracted with ethyl acetate. The organic extracts were dried and filtered and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (petroleum ether to petroleum ether–AcOEt 30%) to yield 5c (227 mg, quantitative). 1H-NMR (CDCl3, 300 MHz) δ 3.78–6.22 (m, 4H), 6.65–8.09 (m, 22H). IR (film) 1649 cm−1. HRMS calcd for C34H26N2O2I2Na: 770.9976. Found: 770.9987. HPLC 5.48 min.N-Benzyl-N-(2-iodophenyl)-2-(2-nitrobenzamido)benzamide 5d
To a solution of 3m (1 g, 2.34 mmol) and pyridine (1 mL, 12.4 mmol) in DCM (15 mL) was added 2-nitrobenzoyl chloride (90%, 486 mg, 2.35 mmol). The mixture was stirred at rt for 24 h and it was basified with NaHCO3 aqueous saturated solution and extracted with DCM. The organic extracts were dried and filtered and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (DCM) to yield 5d (1.14 g, 85%). 1H-NMR (CDCl3, 500 MHz) major conformer δ 4.34 (d, J = 14.3 Hz, 1H), 4.65 (d, J = 14.3 Hz, 1H), 6.75–6.79 (m, 2H), 6.89 (ddd, J = 8, 7.5 and 1.5 Hz, 1H), 7.00 (dd, J = 7.5 and 1.5 Hz, 1H), 7.11 (ddd, J = 8, 7.5 and 1.5 Hz, 1H), 7.15–7.30 (m, 5H), 7.58 (dd, J = 7.5 and 1 Hz, 1H), 7.66 (td, J = 8 and 1.5 Hz, 1H), 7.75 (td, J = 7.5 and 1 Hz, 1H), 7.81 (dd, J = 8 and 1.5 Hz, 1H), 8.13 (d, J = 8.5 Hz, 1H), 8.27 (d, J = 8.5 Hz, 1H), 9.79 (s, 1H). Minor conformer δ 4.61 (d, J = 15 Hz, 1H), 5.25 (d, J = 15 Hz, 1H), other signals masked by the major conformer. Ratio 22 : 1. 13C-NMR (CDCl3, 125 MHz) δ 52.9 (CH2), 99.2 (Cq), 122.6 (CH), 123.1 (CH), 124.4 (Cq), 124.7 (CH), 127.7 (CH), 128.0 (CH), 128.24 (CH), 128.28 (2CH), 129.18 (CH), 129.28 (2CH), 129.34 (CH), 130.7 (CH), 130.9 (CH), 131.5 (CH), 133.0 (Cq), 133.8 (CH), 135.9 (Cq), 136.8 (Cq), 140.2 (CH), 144.2 (Cq), 146.6 (Cq), 164.1 (Cq), 169.6 (Cq). IR (film) 3329, 1688 and 1633 cm−1. HRMS calcd for C27H20N3O4INa: 600.0391. Found: 600.0393.N-Benzyl-N-(2-iodophenyl)-2-(N-methyl-2-nitrobenzamido)benzamide 5e
To a solution of 5d (103 mg, 0.18 mmol), in dry THF (5 mL), were added NaH (60%, 35 mg, 0.87 mmol) and methyl iodide (0.1 mL, 1.60 mmol) and the mixture was stirred overnight at rt under nitrogen. The residue was partitioned between DCM and water, and the aqueous phase was extracted with DCM. The organic extracts were dried and filtered and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (petroleum ether to petroleum ether–AcOEt 50%) to yield 5e (105 mg, quantitative). 1H-NMR (CDCl3, 300 MHz) conformer A δ 3.23 (br s, 3H), 4.53 (d, J = 14.5 Hz, 1H), 5.72 (d, J = 14.5 Hz, 1H), 6.86–7.66 (m, 13H), 6.90 (dd, J = 7.7 and 1.5 Hz, 1H), 7.50 (dd, J = 7.9 and 1 Hz, 1H), 7.94 (dd, J = 7.9 and 1.4 Hz, 1H), 8.25 (dd, J = 7.6 and 1.8 Hz, 1H). Conformer B δ 4.88 (d, J = 15 Hz, 1H), 5.14 (d, J = 15 Hz, 1H), other signals masked by the major conformer. Conformer C δ 4.32 (d, J = 13.8 Hz, 1H), 5.98 (d, J = 13.8 Hz, 1H), other signals masked by the major conformer. Ratio 4.5 : 1 : 1. 13C-NMR (CDCl3, 75.5 MHz) δ 41.2 (CH3), 53.2 (CH2), 98.3 (Cq), 124.5 (CH), 127.0 (CH), 127.3 (CH), 128.2 (3CH), 128.3 (CH), 129.1 (CH), 129.2 (2CH), 129.4 (CH), 129.6 (CH), 131.1 (CH), 133.0 (Cq), 133.5 (Cq), 133.6 (CH), 134.6 (CH), 137.1 (Cq), 139.8 (CH), 141.3 (Cq), 144.5 (Cq), 144.8 (Cq), 167.8 (Cq), 168.3 (Cq). IR (film) 1652 and 1529 cm−1. HRMS calcd for C28H23N3O4I: 592.0728. Found: 592.0734. HPLC 5.59 min.N-(2-Iodophenyl)-N-methyl-2-(2-nitrobenzamido)benzamide 5f
To a solution of 3o (371 mg, 1.05 mmol) and pyridine (0.5 mL, 6.19 mmol) in DCM (5 mL) was added 2-nitrobenzoyl chloride (0.9%, 222 mg, 1.07 mmol). The mixture was stirred overnight at rt and it was basified with NaHCO3 aqueous saturated solution and extracted with DCM. The organic extracts were dried, filtered and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (petroleum ether to petroleum ether–AcOEt 50%) to yield 5f (460 mg, 87%). 1H-NMR (CDCl3, 300 MHz) major conformer δ 3.36 (s, 3H), 6.77 (t, J = 7.5 Hz, 1H), 6.91 (d, J = 8 Hz, 1H), 6.97 (dd, J = 8 and 1.5 Hz, 1H), 7.20–7.29 (m, 3H), 7.63–7.76 (m, 3H), 7.81 (d, J = 8 Hz, 1H), 8.10 (d, J = 8 Hz, 1H), 8.27 (d, J = 8.3 Hz, 1H), 9.97 (br s, 1H). Minor conformer δ 7.14–7.07 (m, 2H), 7.46–7.52 (m, 2H), 7.81–7.96 (m, 1H), 8.39–8.43 (m, 1H), 9.45 (s, 1H), other signals masked by the major conformer. Ratio 6.5 : 1. 13C-NMR (CDCl3, 75.5 MHz) δ 37.8 (CH3), 98.4 (Cq), 122.8 (CH), 123.2 (CH), 124.0 (CH), 124.8 (Cq), 128.6 (CH), 128.7 (CH), 129.2 (CH), 129.8 (CH), 129.9 (CH), 130.8 (CH), 131.0 (CH), 133.0 (Cq), 133.9 (CH), 137.0 (Cq), 140.1 (CH), 146.5 (Cq), 146.7 (Cq), 164.2 (Cq), 170.1 (Cq). IR (film) 3306, 1686, 1631 and 1529 cm−1. HRMS calcd for C21H17N3O4I: 502.0258. Found: 502.0264.N-(2-Iodophenyl)-N-methyl-2-(N-ethyl-2-nitrobenzamido)benzamide 5g
To a solution of 5f (98 mg, 0.2 mmol), in dry THF (5 mL), were added NaH (60%, 39 mg, 0.97 mmol) and ethyl iodide (0.2 mL, 2.48 mmol) and the mixture was stirred at rt under nitrogen overnight. The residue was partitioned between ethyl acetate and water, and the aqueous phase was extracted with ethyl acetate. The organic extracts were dried and filtered and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (petroleum ether to petroleum ether–AcOEt 50%) to yield 5g (93 mg, 90%). 1H-NMR (CDCl3, 300 MHz) conformer A δ 0.86–1.51 (m, 3H), 3.17–3.85 (m, 1H), 3.27 (s, 3H), 4.78–4.93 (m, 1H), 6.80–8.12 (m, 11H), 8.28–8.32 (m, 1H). Conformer B δ 3.42 (s, 3H), other signals masked by major conformer. Conformer C δ 3.23 (s, 3H), other signals masked by major conformer. Conformer D δ 3.46 (s, 3H), other signals masked by major conformer. Ratio 3.2 : 2.5 : 1.5 : 1. IR (film) 1649 and 1528 cm−1. HRMS calcd for C23H21N3O4I: 530.0571. Found: 530.0560. HPLC 4.49 min.N-Ethyl-N-(2-iodophenyl)-2-(2-nitrobenzamido)benzamide 5h
To a solution of 3n (417 mg, 1.14 mmol) and pyridine (0.5 mL, 6.19 mmol) in DCM (5 mL) was added 2-nitrobenzoyl chloride (0.9%, 260 mg, 1.26 mmol). The mixture was stirred at rt overnight and it was basified with NaHCO3 aqueous saturated solution and extracted with DCM. The organic extracts were dried and filtered and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (petroleum ether to petroleum ether–AcOEt 50%) to yield 5h (524 mg, 89%). 1H-NMR (CDCl3, 300 MHz) major conformer δ 1.18 (t, J = 7.2 Hz, 3H), 3.53 (dq, J = 7.2 and 6.9 Hz, 1H), 4.25 (dq, J = 7.2 and 6.9 Hz, 1H), 6.77 (ddd, J = 7.8, 7.5 and 1.2 Hz, 1H), 6.92 (dd, J = 7.5 and 1.2 Hz, 1H), 6.97 (dd, J = 7.5 and 1.2 Hz, 1H), 7.14 (dd, J = 7.8 and 1.5 Hz, 1H), 7.21–7.31 (m, 2H), 7.61–7.79 (m, 3H), 7.84 (dd, J = 7.8 and 1.2 Hz, 1H), 8.13 (d, J = 8.1 Hz, 1H), 8.26 (d, J = 8.1 Hz, 1H), 9.91 (br s, 1H). Minor conformer δ 1.15–1.21 (m, 1H, masked by the major conformer), 3.63–3.77 (m, 1H), 3.81–3.95 (m, 1H), 6.77–8.30 (m, 12H, masked by the major conformer), 9.22 (br s, 1H). Ratio 5.5 : 1. 13C-NMR (CDCl3, 75.5 MHz) δ 12.1 (CH3), 44.8 (CH2), 99.4 (Cq), 122.8 (CH), 123.2 (CH), 124.6 (Cq), 124.8 (CH), 128.3 (CH), 128.7 (CH), 129.2 (CH), 129.5 (CH), 130.8 (CH), 130.9 (CH), 131.2 (CH), 133.1 (Cq), 134.1 (CH), 136.9 (Cq), 140.3 (CH), 144.4 (Cq), 146.4 (Cq), 165.0 (Cq), 170.6 (Cq). IR (film) 3307, 1682 and 1632 cm−1. HRMS calcd for C22H19N3O4I: 516.0415. Found: 516.0406.N-Ethyl-N-(2-iodophenyl)-2-(N-methyl-2-nitrobenzamido)benzamide 5i
To a solution of 5h (99 mg, 0.19 mmol), in dry THF (5 mL), were added NaH (60%, 43 mg, 1.07 mmol) and methyl iodide (0.15 mL, 2.40 mmol) and the mixture was stirred overnight at rt under nitrogen. The residue was partitioned between ethyl acetate and water, and the aqueous phase was extracted with ethyl acetate. The organic extracts were dried and filtered and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (petroleum ether to petroleum ether–AcOEt 50%) to yield 5i (102 mg, quantitative). 1H-NMR (CDCl3, 300 MHz) conformer A δ 1.16 (t, J = 6.9 Hz, 3H), 3.28 (s, 3H), 3.66 (dq, J = 7.2 and 6.9 Hz, 1H), 4.58 (dq, J = 7.2 and 6.9 Hz, 1H), 6.76–7.68 (m, 8H), 7.79–8.01 (m, 3H), 8.22–8.27 (m, 1H). Conformer B δ 1.12 (t, J = 7.2 Hz, 3H), 3.20 (s, 3H), 3.53–3.60 (m, 1H), 3.95 (dq, J = 7.5 and 7.2 Hz, 1H), 6.76–8.27 (m, 12H, masked by the major conformer). Conformer C δ 3.52 (s, 3H), other signals masked by major conformer. Ratio 4.5 : 2 : 1. IR (film) 1650 and 1529 cm−1. HRMS calcd for C23H21N3O4I: 530.0571. Found: 530.0577.N-(2-Iodophenyl)-N-methyl-2-(N-methyl-2-nitrobenzamido)benzamide 5j
To a solution of 5f (94 mg, 0.19 mmol), in dry THF (5 mL), were added NaH (60%, 38 mg, 0.95 mmol) and methyl iodide (0.15 mL, 2.41 mmol) and the mixture was stirred overnight at rt under nitrogen. The residue was partitioned between ethyl acetate and water, and the aqueous phase was extracted with ethyl acetate. The organic extracts were dried and filtered and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (petroleum ether to petroleum ether–AcOEt 50%) to yield 5j (54 mg, 56%). 1H-NMR (CDCl3, 300 MHz) conformer A δ 3.28 (s, 3H), 3.43 (s, 3H), 6.83–8.27 (m, 12H). Conformer B δ 3.22 (s, 3H), 3.31 (s, 3H), 6.83–8.27 (m, 12H). Conformer C δ 3.46 (s, 3H), 3.58 (s, 3H), 6.83–8.27 (m, 12H). Ratio 4.2 : 4 : 1. IR (film) 1657, 1650 and 1529 cm−1. HRMS calcd for C22H19N3O4I: 516.0415. Found: 516.0411.2-Benzamido-N-(2-iodophenyl)-N-methylbenzamide 5k
To a solution of 3o (399 mg, 1.13 mmol) and pyridine (0.5 mL, 6.19 mmol) in DCM (5 mL) was added benzoyl chloride (0.2 mL, 1.72 mmol). The mixture was stirred at rt overnight and it was basified with NaHCO3 aqueous saturated solution and extracted with DCM. The organic extracts were dried, filtered and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (petroleum ether to petroleum ether–AcOEt 50%) to yield 5k (335 mg, 65%). 1H-NMR (CDCl3, 500 MHz) major conformer δ 3.41 (s, 3H), 6.71 (t, J = 7.5 Hz, 1H), 6.91 (t, J = 7.5 Hz, 1H), 6.97 (d, J = 7.5 Hz, 1H), 7.10 (d, J = 7.3 Hz, 1H), 7.23–7.29 (m, 2H), 7.52–7.57 (m, 3H), 7.78 (d, J = 7.7 Hz, 1H), 8.04 (d, J = 7 Hz, 2H), 8.48 (d, J = 8.3 Hz, 1H), 10.70 (s, 1H). Minor conformer δ 3.34 (s, 3H), 7.65–7.70 (m, 1H), 7.94–8.00 (m, 2H), 8.50–8.57 (m, 1H), 10.05 (s, 1H), other signals masked by the major conformer. Ratio 4.5 : 1. 13C-NMR (CDCl3, 125 MHz) δ 38.0 (CH3), 98.5 (Cq), 121.9 (CH), 122.1 (CH), 122.9 (Cq), 127.3 (2CH), 128.8 (2CH), 129.0 (CH), 129.1 (CH), 129.57 (CH), 129.60 (CH), 131.1 (CH), 131.9 (CH), 134.6 (Cq), 138.3 (Cq), 140.3 (CH), 146.7 (Cq), 165.0 (Cq), 170.6 (Cq). IR (film) 3337, 1676 and 1634 cm−1. HRMS calcd for C21H18N2O2I: 457.0407. Found: 457.0406.2-(N-Benzylbenzamido)-N-(2-iodophenyl)-N-methylbenzamide 5l
To a solution of 5k (93 mg, 0.20 mmol), in dry THF (5 mL), were added NaH (60%, 30 mg, 0.75 mmol) and benzyl bromide (0.1 mL, 0.84 mmol) and the mixture was stirred overnight at rt under nitrogen. The mixture was partitioned between ethyl acetate and water, and the aqueous phase was extracted with ethyl acetate. The organic extracts were dried and filtered and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (petroleum ether to petroleum ether–AcOEt 50%) to yield 5l (81 mg, 73%). 1H-NMR (CDCl3, 300 MHz) conformer A δ 3.25 (s, 3H), 4.24 (d, J = 15 Hz, 1H), 6.05 (d, J = 15 Hz, 1H), 6.55–7.97 (m, 18H). Conformer B δ 3.42 (s, 3H) other signals masked by major conformer. Other conformers masked. Ratio 1.33 : 1 : ?. IR (film) 1651 cm−1. HRMS calcd for C28H23N2O2INa: 569.0696. Found: 569.0697. HPLC 5.45 min.2-(N-Ethylbenzamido)-N-(2-iodophenyl)-N-methylbenzamide 5m
To a solution of 5k (90 mg, 0.197 mmol), in dry THF (5 mL), were added NaH (60%, 45 mg, 1.125 mmol) and ethyl iodide (0.2 mL, 2.49 mmol) and it was stirred at rt under nitrogen overnight. The mixture was partitioned between ethyl acetate and water, and the aqueous phase was extracted with ethyl acetate. The organic extracts were dried and filtered and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (petroleum ether to petroleum ether–AcOEt 50%) to yield 5m (67 mg, 70%). 1H-NMR (CDCl3, 300 MHz) conformer A δ 1.25 (t, J = 6.9 Hz, 3H), 3.23 (s, 3H), 3.60–3.90 (m, 2H), 6.54–7.98 (m, 12H). Conformer B δ 3.36 (s, 3H), 4.34–4.51 (m, 1H), 4.60–4.74 (m, 1H) other signals masked by the major conformer. Other conformers masked. Ratio 2 : 1 : ?. IR (film) 1647 cm−1. HRMS calcd for C23H21N2O2INa: 507.0540. Found: 507.0542. HPLC 5.48 min.2-Benzamido-N-benzyl-N-phenylbenzamide 5n
To a solution of 4g (780 mg, 2.58 mmol) and pyridine (1 mL, 12.4 mmol) in DCM (10 mL) was added benzoyl chloride (0.4 mL, 3.44 mmol). The mixture was stirred overnight at rt and it was basified with NaHCO3 aqueous saturated solution and extracted with DCM. The organic extracts were dried and filtered and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (petroleum ether to petroleum ether–AcOEt 50%) to yield 5n (1.03 g, 98%). 1H-NMR (CDCl3, 500 MHz) major conformer δ 5.16 (s, 2H), 6.71 (ddd, J = 8, 7.5 and 1 Hz, 1H), 6.87–6.90 (m, 2H), 6.93 (dd, J = 7.5 and 1 Hz, 1H), 7.09–7.17 (m, 3H), 7.21–7.23 (m, 3H), 7.25–7.30 (m, 3H), 7.52 (dd, J = 7.5 and 7 Hz, 2H), 7.58 (ddd, J = 7.5, 7 and 1.5 Hz, 1H), 7.98 (d, J = 8.5 Hz, 1H), 8.40 (dd, J = 8.5 and 1 Hz, 1H), 10.51 (s, 1H). Minor conformer δ 7.46 (dd, J = 8 and 7.5 Hz, 1H), 8.10 (dd, J = 8.5 and 1 Hz, 1H), other signals masked by the major conformer. Ratio 8 : 1. 13C-NMR (CDCl3, 125 MHz) δ 54.0 (CH2), 121.9 (CH), 122.4 (CH), 123.8 (Cq), 127.06 (CH), 127.16 (2CH), 127.23 (2CH), 127.5 (CH), 128.0 (2CH), 128.5 (2CH), 128.8 (2CH), 129.2 (2CH), 129.3 (CH), 130.8 (CH), 131.9 (CH), 134.4 (Cq), 136.9 (Cq), 138.0 (Cq), 143.1 (Cq), 165.1 (Cq), 170.2 (Cq). IR (film) 3332, 1677 and 1632 cm−1. HRMS calcd for C27H23N2O2: 407.1754. Found: 407.1752.N-Benzyl-2-(N-benzylbenzamido)-N-phenylbenzamide 5o
To a solution of 5n (205 mg, 0.50 mmol), in dry THF (5 mL), were added NaH (60%, 80 mg, 2 mmol) and benzyl bromide (0.3 mL, 2.51 mmol) and it was stirred overnight at rt under nitrogen. The mixture was partitioned between ethyl acetate and brine, and the aqueous phase was extracted with ethyl acetate. The organic extracts were dried and filtered and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (petroleum ether to AcOEt) to yield 5o (188 mg, 75%). 1H-NMR (CDCl3, 300 MHz) δ 4.10 (d, J = 15 Hz, 1H), 5.10–5.30 (m, 2H), 5.97 (d, J = 15 Hz, 1H), 6.54–7.70 (m, 24H). IR (film) 1644 cm−1. HRMS calcd for C34H28N2O2Na: 519.2043. Found: 519.2044. HPLC 5.42 min.Attempted synthesis of ethyl 2-(2-[N-benzyl-2-iodobenzamido]benzamido)benzoate
A solution of 3i (200 mg, 0.44 mmol) in thionyl chloride (5 mL) was refluxed for 3 h. The excess of thionyl chloride was removed under reduced pressure and the residue was dissolved in AcOEt (10 mL). The solution was cooled to 0 °C and Et3N (0.5 mL, 3.59 mmol) and ethyl 2-aminobenzoate (65 µL, 0.44 mmol) were added. The mixture was allowed to warm to rt and stirred overnight. The mixture was partitioned between ethyl acetate and aqueous NaHCO3 saturated solution, and the aqueous phase was extracted with ethyl acetate. The organic extracts were dried and filtered and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (DCM) to yield 2-(2-iodophenyl)-4H-benzo[d][1,3]oxazin-4-one 6a (114 mg, 75%). 1H-NMR (CDCl3, 300 MHz) δ 7.24 (ddd, J = 7.8, 7.5 and 1.8 Hz, 1H), 7.53 (td, J = 7.5 and 1.2 Hz, 1H), 7.63 (td, J = 7.5 and 1.2 Hz, 1H), 7.79 (dd, J = 8.1 and 1.2 Hz, 1H), 7.89 (td, J = 7.8 and 1.8 Hz, 1H), 7.91 (td, J = 8.1 and 1.5 Hz, 1H), 8.08 (dd, J = 7.8 and 1.2 Hz, 1H), 8.33 (dd, J = 7.8 and 1.5 Hz, 1H). 13C-NMR (CDCl3, 75.5 MHz) δ 94.6 (Cq), 116.9 (Cq), 127.3 (CH), 128.2 (CH), 128.6 (CH), 129.0 (CH), 130.9 (CH), 132.3 (CH), 135.5 (Cq), 136.7 (CH), 141.1 (CH), 146.2 (Cq), 157.8 (Cq), 159.2 (Cq). IR (film) 1764 cm−1. HRMS calcd for C14H9NO2I: 349.9672. Found: 349.9672. Anal. calcd for C14H8NO2I (349.1) C, 48.16; H, 2.31; N, 4.01. Found: C, 48.59; H, 2.30; N, 3.96%. Mp 120–122 °C.Attempted synthesis of ethyl 2-(2-[N-benzyl-1-naphthamido]benzamido)benzoate
A solution of 3g (468 mg, 1.23 mmol) in thionyl chloride (10 mL) was refluxed for 3 h. The excess of thionyl chloride was removed under reduced pressure and the residue was dissolved in AcOEt (12 mL). The solution was cooled to 0 °C and Et3N (2.6 mL, 18.6 mmol) and ethyl 2-aminobenzoate (0.18 mL, 1.22 mmol) were added. The mixture was allowed to warm to rt and stirred overnight. The mixture was partitioned between ethyl acetate and aqueous NaHCO3 saturated solution, and the aqueous phase was extracted with ethyl acetate. The organic extracts were dried and filtered and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (DCM) to yield 2-(naphthalen-4-yl)-4H-benzo[d][1,3]oxazin-4-one 6b (289 mg, 86%). 1H-NMR (CDCl3, 300 MHz) δ 7.53–7.61 (m, 3H), 7.66 (ddd, J = 8.4, 6.9 and 1.5 Hz, 1H), 7.79 (dd, J = 8.1 and 1.2 Hz, 1H), 7.85 (dd, J = 6.9 and 1.5 Hz, 1H), 7.92 (dd, J = 8.1 and 1.5 Hz, 1H), 8.04 (d, J = 8.1 Hz, 1H), 8.29 (dd, J = 7.8 and 1.2 Hz, 1H), 8.31 (dd, J = 7.5 and 1.2 Hz, 1H), 9.15 (d, J = 8.4 Hz, 1H). 13C-NMR (CDCl3, 75.5 MHz) δ 116.8 (Cq), 124.7 (CH), 125.7 (CH), 126.3 (CH), 126.8 (Cq), 127.3 (CH), 127.8 (CH), 128.46 (CH), 128.54 (CH), 128.8 (CH), 130.0 (CH), 130.7 (Cq), 133.1 (CH), 134.0 (Cq), 136.5 (CH), 146.7 (Cq), 157.6 (Cq), 159.7 (Cq). IR (film) 1762 cm−1. HRMS calcd for C18H12NO2: 274.0863. Found: 274.0863. Mp 108–130 °C.N,N′-Di(2-iodobenzoyl)benzene-1,2-diamine 7a
To a solution of 1,2-diaminobenzene (255 mg, 2.36 mmol) and pyridine (1 mL, 12.4 mmol) in DCM (10 mL), was added 2-iodobenzoyl chloride (1.24 g, 4.65 mmol). The mixture was stirred at rt under nitrogen for 24 h. To the resulting suspension petroleum ether was added and the mixture was filtrated to obtain 7a as an unsoluble solid (1 g, 75%) which was used without further purification. m/z (ES−) 567 (100%, M–H+).N,N′-Di(2-iodobenzoyl)-N,N′-dimethylbenzene-1,2-diamine 7b
To a suspension of 7a (102 mg, 0.18 mmol) in THF (25 mL), was added NaH (60%, 60 mg, 1.5 mmol), and methyl iodide (0.50 mL, 8.04 mmol). The mixture was stirred at rt under nitrogen for 24 h. The residue was partitioned between DCM and water, and the aqueous phase was extracted with DCM. The organic extracts were dried and filtered and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (petroleum ether to petroleum ether–AcOEt 50%) to yield 7b (91 mg, 85%). 1H-NMR (CDCl3, 300 MHz) major conformer δ 3.26 (s, 3H), 7.14 (ddd, J = 7.8, 7.5 and 1.8 Hz, 2H), 7.33–7.37 (m, 2H), 7.44 (dd, J = 7.2 and 0.9 Hz, 2H), 7.46–7.52 (m, 2H), 7.57–7.60 (m, 2H), 7.91 (d, J = 7.8 Hz, 2H). Minor conformer δ 3.57 (s, 3H), 6.88 (td, J = 7.8 and 1.5 Hz, 2H), 7.04–7.92 (m, 8H, masked by the major conformer), 7.79 (d, J = 7.8 Hz, 2H). Ratio 5 : 1. 13C-NMR (CDCl3, 75.5 MHz) δ 39.9 (CH3), 92.4 (Cq), 126.9 (CH), 128.4 (CH), 129.3 (CH), 130.5 (CH), 130.6 (Cq), 139.5 (CH), 142.1 (Cq), 170.5 (Cq). IR (film) 1649 cm−1. HRMS calcd for C22H19N2O2I2: 596.9530. Found: 596.9530. HPLC 5.50 min.N,N′-Dibenzyl-N,N′-di(2-iodobenzoyl)benzene-1,2-diamine 7c
To a suspension of 7a (126 mg, 0.22 mmol) in THF (25 mL), were added NaH (60%, 96 mg, 2.4 mmol), and benzyl bromide (0.25 mL, 2.09 mmol). The mixture was stirred at rt under nitrogen for 24 h. The residue was partitioned between DCM and water, and the aqueous phase was extracted with DCM. The organic extracts were dried and filtered and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (petroleum ether to petroleum ether–AcOEt 50%) to yield 7c (132 mg, 79%). 1H-NMR (CDCl3, 300 MHz) δ 4.33–4.97 (m, 4H), 5.95–6.05 (m, 1H), 6.56–7.93 (m, 21H). Complex mixture of conformers. IR (film) 1649 cm−1. HRMS calcd for C34H27N2O2I2: 749.0156. Found: 749.0154.X-Ray crystallography
Data for the X-ray crystal structures described in this paper have been deposited with the Cambridge Crystallographic database.†Acknowledgements
We are grateful to the EU Marie Curie programme, MEC (Spain, EX2004-0501), the Leverhulme Trust and the EPSRC for funds.References
- J. Clayden, Angew. Chem., Int. Ed. Engl., 1997, 36, 949–951 CrossRef CAS.
- J. Clayden, Synlett, 1998, 810–816 CrossRef CAS.
- (a) P. Bowles, J. Clayden and M. Tomkinson, Tetrahedron Lett., 1995, 36, 9219–9222 CrossRef CAS; (b) J. Clayden, N. Westlund and F. X. Wilson, Tetrahedron Lett., 1996, 37, 5577–5580 CrossRef CAS; (c) P. Bowles, J. Clayden, M. Helliwell, C. McCarthy, M. Tomkinson and N. Westlund, J. Chem. Soc., Perkin Trans. 1, 1997, 2607–2616 RSC; (d) J. Clayden and J. H. Pink, Tetrahedron Lett., 1997, 38, 2561–2564 CrossRef CAS; (e) J. Clayden, M. Darbyshire, J. H. Pink, N. Westlund and F. X. Wilson, Tetrahedron Lett., 1997, 38, 8587–8590 CrossRef CAS; (f) J. Clayden, J. H. Pink and S. A. Yasin, Tetrahedron Lett., 1998, 39, 105–108 CrossRef CAS; (g) J. Clayden and J. H. Pink, Angew. Chem., Int. Ed., 1998, 37, 1937–1939 CrossRef CAS; (h) A. Ahmed, R. A. Bragg, J. Clayden, L. W. Lai, C. McCarthy, J. H. Pink, N. Westlund and S. A. Yasin, Tetrahedron, 1998, 54, 13277–13394 CrossRef CAS; (i) J. Clayden, N. Westlund and F. X. Wilson, Tetrahedron Lett., 1999, 40, 3329–3330 CrossRef CAS; (j) J. Clayden and L. W. Lai, Angew. Chem., Int. Ed., 1999, 38, 2556–2558 CrossRef CAS; (k) J. Clayden, N. Westlund and F. X. Wilson, Tetrahedron Lett., 1999, 40, 7883–7887 CrossRef CAS; (l) R. A. Bragg and J. Clayden, Tetrahedron Lett., 1999, 40, 8327–8331 CrossRef CAS; (m) J. Clayden, C. McCarthy and J. G. Cumming, Tetrahedron Lett., 2000, 41, 3279–3283 CrossRef CAS; (n) J. Clayden, N. Westlund, R. L. Beddoes and M. Helliwell, J. Chem. Soc., Perkin Trans. 1, 2000, 1351–1361 RSC; (o) J. Clayden, C. McCarthy, N. Westlund and C. S. Frampton, J. Chem. Soc., Perkin Trans. 1, 2000, 1363–1378 RSC; (p) J. Clayden, N. Westlund and C. S. Frampton, J. Chem. Soc., Perkin Trans. 1, 2000, 1379–1385 RSC; (q) J. Clayden, P. Johnson, J. H. Pink and M. Helliwell, J. Org. Chem., 2000, 65, 7033–7040 CrossRef CAS; (r) J. Clayden, M. Helliwell, C. McCarthy and N. Westlund, J. Chem. Soc., Perkin Trans. 1, 2000, 3232–3249 RSC; (s) J. Clayden, P. Johnson and J. H. Pink, J. Chem. Soc., Perkin Trans. 1, 2001, 371–375 RSC; (t) J. Clayden, L. W. Lai and M. Helliwell, Tetrahedron: Asymmetry, 2001, 12, 695–698 CrossRef CAS; (u) J. Clayden, Chem. Commun., 2004, 127–135 RSC; (v) J. Clayden, C. C. Stimson, M. Keenan and A. E. H. Wheatley, Chem. Commun., 2004, 228–229 RSC.
- R. Adams and N. K. Sundholm, J. Am. Chem. Soc., 1948, 70, 2667–2673 CrossRef CAS.
- W. E. Stewart and T. H. Siddall, III, Chem. Rev., 1970, 70, 517–551 CrossRef.
- B. F. Pedersen and B. Pedersen, Tetrahedron Lett., 1965, 6, 2995–3001 CrossRef.
- (a) A. Itai, Y. Toriumi, S. Saito, H. Kagechika and K. Shudo, J. Am. Chem. Soc., 1992, 114, 10649–10650 CrossRef CAS; (b) I. Azumaya, H. Kagechika, Y. Fujiwara, M. Itoh, K. Yamaguchi and K. Shudo, J. Am. Chem. Soc., 1991, 113, 2833 CrossRef CAS.
- (a) D. P. Curran, H. Qi, S. J. Geib and N. C. DeMello, J. Am. Chem. Soc., 1994, 116, 3131–3132 CrossRef CAS; (b) A. D. Hughes and N. S. Simpkins, Synlett, 1998, 967–968 CrossRef CAS; (c) O. Kitagawa, H. Izawa, K. Sato, A. Dobashi and T. Taguchi, J. Org. Chem., 1998, 63, 2634–2640 CrossRef CAS; (d) D. P. Curran, W. Liu and C. H. Chen, J. Am. Chem. Soc., 1999, 121, 11012–11013 CrossRef CAS; (e) O. Kitagawa, S. Momose, Y. Fushimi and T. Taguchi, Tetrahedron Lett., 1999, 40, 8827–8831 CrossRef CAS; (f) A. D. Hughes, D. A. Price and N. S. Simpkins, J. Chem. Soc., Perkin Trans. 1, 1999, 1295–1304 RSC; (g) T. Bach, J. Schröder and K. Harms, Tetrahedron Lett., 1999, 40, 9003–9004 CrossRef CAS; (h) O. Kitagawa, M. Kohriyama and T. Taguchi, J. Org. Chem., 2002, 67, 8682–8684 CrossRef CAS; (i) J. Terauchi and D. P. Curran, Tetrahedron: Asymmetry, 2003, 14, 587–592 CrossRef CAS; (j) D. P. Curran, C. H.-T. Chen, S. J. Geib and A. J. B. Lapierre, Tetrahedron, 2004, 60, 4413–4424 CrossRef CAS; (k) M. Petit, S. J. Geib and D. P. Curran, Tetrahedron, 2004, 60, 7543–7552 CrossRef CAS.
- T. Adler, J. Bonjoch, J. Clayden, M. Font-Bardía, M. Pickworth, X. Solans, D. Solé and L. Vallverdú, Org. Biomol. Chem., 2005, 3, 3173–3183 RSC.
- Previous reports have addressed the conformation of constrained, cyclic benzanilides: (a) S. J. Edge, W. D. Ollis, J. S. Stephanatou and J. F. Stoddart, J. Chem. Soc., Perkin Trans. 1, 1982, 1701–1714 RSC; (b) A. Hoorfar, W. D. Ollis and J. F. Stoddart, J. Chem. Soc., Perkin Trans. 1, 1982, 1721–1726 RSC.
- For a discussion of intramolecular conformational interactions between amide groups see: (a) J. Clayden, A. Lund, L. Vallverdú and M. Helliwell, Nature, 2004, 431, 966–971 CrossRef CAS; (b) J. Clayden, A. Lund and L. H. Youssef, Org. Lett., 2001, 3, 4133–4136 CrossRef CAS; (c) J. Clayden, M. N. Kenworthy and L. H. Youssef, Tetrahedron Lett., 2000, 41, 5171–5174 CrossRef CAS; (d) J. Clayden, N. Westlund and F. X. Wilson, Tetrahedron Lett., 1999, 40, 3331–3334 CrossRef CAS and ref. 3f, 3g, 3t.
- A. Tanatani, A. Yokoyama, I. Azumaya, Y. Takakura, C. Mitsui, M. Shiro, M. Uchiyama, A. Muranaka, N. Kobayashi and T. Yokozawa, J. Am. Chem. Soc., 2005, 127, 8553–8561 CrossRef CAS.
- T. Nishimura, K. Maeda and E. Yashima, Chirality, 2004, 16, S12–S22 CrossRef CAS.
- See also: M. S. Betson, J. Clayden, H. K. Lam and M. Helliwell, Angew. Chem., Int. Ed., 2005, 44, 1241–1244 Search PubMed.
- M. S. Betson, J. Clayden, M. Helliwell, M. N. Kenworthy, L. W. Lai, H. K. Lam, A. Lund, L. Vallderdú, S. A. Yasin and L. H. Youssef, manuscript in preparation.
- Z. Yin, Z. Li, A. Yu, J. He and J.-P. Cheng, Tetrahedron Lett., 2004, 45, 6803–6808 CrossRef CAS.
- I. Azumaya, I. Okamoto, S. Nakayama, A. Tanatani, K. Yamaguchi, K. Shudo and H. Kagechika, Tetrahedron, 1999, 55, 11237–11246 CrossRef CAS.
- I. Azumaya, Y. Sagara and H. Takayanagi, Anal. Sci., 2002, 18, 1403–1404 CrossRef CAS.
- Two enantiomeric conformations are possible for these compounds. Crystallization of 7a in both a racemic mixture or as a single enantiomer was previously reported: see ref. 16.
- R. M. Baldwin, T.-H. Lin and H. S. Winchell, Eur. Pat., EP ( 1980) 11858 19800611 (Chem. Abs., 94, 83749) Search PubMed.
Footnote |
| † CCDC reference numbers 299474 (3a); 299475 (3b); 299476 (7b). For crystallographic data in CIF or other electronic format see DOI: 10.1039/b602912d |
| This journal is © The Royal Society of Chemistry 2006 |
