The small peptide-catalyzed direct asymmetric aldol reaction in water†
Pawel Dziedzica, Weibiao Zoua, Jonas Háfrenb and Armando Córdova*a
aDepartment of Organic Chemistry, Arrhenius Laboratory, Stockholm University, Sweden. E-mail: acordova1a@netscape.net; acordova@organ.su.se; Fax: + 46 8 15 49 08; Tel: +46 8 162479
bSwedish University of Agricultural Sciences, Department of Wood Science, Box 7008, SE-750 07, Uppsala, Sweden
First published on 22nd November 2005
Abstract
Simple modular di- and tripeptides with a primary amine at the N-terminus catalyze the aqueous asymmetric aldol reaction between unmodified ketones and aldehydes to furnish the corresponding β-hydroxy ketones with up to 86% ee in water and 99% ee in aqueos media.
The asymmetric aldol reaction is a powerful method for forming carbon–carbon bonds.1,2 In Nature, there are two types of aldolase enzymes that catalyze the direct aldol reaction with excellent stereocontrol: class I aldolases employ chiral enamines whereas class II aldolases utilizes chiral Zn enolates as nucleophiles for the stereoselective addition of dihydroxyacetone phosphate (DHAP) to aldehydes.1 Water is the reaction media for most enzymatic reactions in living systems. Thus, the stereoselective aldol reaction plausibly evolved in aqueous media along with the evolution of aldolase enzymes. In organic synthesis, there are several methods for achieving directed catalytic stereoselective aldol reactions.2,3 However, methods for catalyzing enantioselective aldol reactions in water are rare.4 For example, chiral organometallic complexes have been developed that catalyze the asymmetric Mukaiyama-type aldol reaction between activated silyl enol ethers and aldehydes in aqueous media.5 Moreover, biocatalysts mediate the direct asymmetric aldol reaction with high stereoselectivity in aqueous buffers.1,6,7 Organocatalysis is an active research area.8 In this context, proline and its derivatives are excellent catalysts for the direct asymmetric aldol reaction in organic solvent.9 However, proline and other chiral pyrrolidine derivatives furnish nearly racemic products in water.10 Moreover, amino acids catalyze the asymmetric dimerization of glycol aldehyde to furnish tetroses with low ees in water.11 This has led to speculation that amino acid catalysis is a plausible mechanism for the origins of homochirality. We recently found that acyclic amino acids12a and small linear peptides12b catalyze the direct intermolecular asymmetric aldol reaction with high stereoselectivity in organic solvents. We therefore wanted to expand this concept to water or aqueous media providing for the asymmetric assembly of aldol products under environmentally benign reaction conditions. Moreover, the lessons learnt from these studies would give important information on a molecular level about the evolution of homochirality and catalysis of aldolase enzymes. Herein, we report that small peptides with a primary amine as the catalytic residue, catalyze the asymmetric aqueous aldol reaction between unmodified ketones and aldehydes to furnish the corresponding aldol products with high ees (up to 86% in H2O, up to 99% in aqueous media). In stark contrast, natural amino acids catalyzed the formation of nearly racemic aldol products, which implies that peptide bond-formation is important for the evolution of high asymmetry in water.
In initial experiments, we screened a variety of natural amino acids, di- and tripeptides for their ability to catalyze the asymmetric aldol reaction between cyclohexanone 1a (0.75 mmol) and p-nitrobenzaldehyde (0.25 mmol) in water (1 mL) and sodium dodecyl sulfate (SDS, 0.25 mmol) (Table 1).
| |||||
|---|---|---|---|---|---|
| Entry | Catalyst | Time/h | Yield (%)a | Drb | Ee (%)c |
| a Isolated yield after silica-gel column chromatography.b anti : syn ratio as determined by NMR analyses of the crude product.c Determined by chiral-phase HPLC analyses.d 20 vol% DMF and no SDS. | |||||
| 1 | L-val–L-val | 76 | 40 | 2 : 1 | 67 |
| 2 | L-val–L-phe | 52 | 47 | 3 : 1 | 83 |
| 3 | L-val–L-phe | 70d | 70 | 2 : 1 | 83 |
| 4 | L-val–L-ala | 96 | 30 | 2 : 1 | 71 |
| 5 | L-val–L-ala | 68 | 22 | 2 : 1 | 70 |
| 6 | L-val–L-val | 68 | 45 | 2 : 1 | 56 |
| 7 | L-ala–L-ala–L-ala | 120 | 42 | 2 : 1 | 75 |
| 8 | L-ser–L-ala | 73 | 80 | 2 : 1 | 39 |
| 9 |  | 96 | 40 | 1 : 1 | 67 |
| 10 | L-valine | 41 | 20 | 1 : 1 | <5 |
| 11 | L-proline | 42 | 10 | 1 : 1 | <5 |
| 12 | L-alanine | 163 | 12 | 1 : 1 | 0 |
We found a remarkable high difference in stereoselectivity between the small peptide- and amino acid-catalyzed direct aldol reactions: All the peptides tested catalyzed the asymmetric formation of the desired aldol product 2a with 39–83% ee whereas the simple amino acids catalyzed the formation of small amounts of 2a with <5% ee. For example, L-vaL–L-phe and (L-ala)3 mediated the asymmetric assembly of product 2a in 47% yield with 3 : 1 dr and 83% ee and 42% yield with 2 : 1 dr and 75% ee, respectively. Moreover, the tripeptide (L-ala)3 catalyzed the asymmetric assembly of 2a with a higher ee as compared to the dipeptide (L-ala)2 showing the beneficial effect of structural complexity in water. In addition, valine tetrazole 3 catalyzed the asymmetric formation of 2a with 1 : 1 dr and 67% ee. Hence, converting amino acids to tetrazole derivatives can have a beneficial effect in water as well as in organic solvent.9o The addition of a small amount of DMF (20 vol%) instead of SDS increased the yields and ees of 2a. Thus, we also investigated the peptide-catalyzed direct asymmetric aldol reaction in different aqueous buffer and media (Table 2).
| |||||
|---|---|---|---|---|---|
| Entry | Catalyst | Solvent | Yield (%)a | Drb | Ee (%)c |
| a Isolated yield after silica-gel column chromatography.b anti : syn ratio as determined by NMR analyses of the crude product.c Determined by chiral-phase HPLC analyses.d Phosphate buffer, 40 mM, pH 7.2, SDS.e NaOAc buffer, 0.1M, pH 4.2, SDS.f 1 equiv. SDS.g 1 equiv. α-cyclodextrin added. | |||||
| 1 | L-val–L-phe | Phosphate bufferd | 48 | 2 : 1 | 83 |
| 2 | L-val–L-phe | NaOAc buffere | 27 | 2 : 1 | 85 |
| 3 | L-val–L-phe | H2Of | 47 | 3 : 1 | 83 |
| 4 | L-val–L-phe | H2Og | 48 | 3 : 1 | 86 |
| 5 | L-val–L-phe | H2O–DMF, 5 : 1 | 70 | 2 : 1 | 83 |
| 6 | L-val–L-ala | H2O–DMF, 1 : 1 | 67 | 3 : 1 | 93 |
| 7 | L-ala–L-ala | H2O–EtOH, 1 : 1 | 72 | 3 : 1 | 83 |
| 8 | L-ser–L-phe | H2O–MeOH, 1 : 1 | 90 | 2 : 1 | 81 |
| 9 | L-ala–L-ala | H2O–NMP, 1 : 1 | 56 | 3 : 1 | 87 |
| 10 | L-ala–L-ala | H2O–DMSO, 1 : 1 | 80 | 3 : 1 | 86 |
The enantioselectivity of the dipeptide-catalyzed aldol reaction was not affected by performing the reactions in buffered aqueous media and product 2a was formed with high ees. However, the efficiency of the peptide-catalyzed asymmetric aldol reactions decreased at low pH. Inspired by the pioneering studies of Breslow and co-workers,13 we also investigated whether the addition of α-cyclodextrin to the peptide-catalyzed asymmetric aldol reaction in water (no SDS) would improve the enantioselectivity by creating a hydrophobic environment (entry 4). In fact, L-val–L-phe–L-ala catalyzed the asymmetric assembly of 2a with 3 : 1 dr and 86% ee under these reaction conditions. The dipetide-catalyzed direct asymmetric aldol reaction proceeded smoothly in aqueous media and product 2a was isolated in high yield with 3 : 1 dr and up to 93% ee. Encouraged by these results we also investigated the peptide-catalyzed aqueous asymmetric aldol reaction for a set of different ketones and aldehydes in aqueous media (Table 3).
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Dipeptide | Ketone | R | Product | Conditions | Yield (%)a | Drb | Ee (%)c |
| a Isolated yield after silica-gel column chromatography.b anti : syn ratio as determined by NMR analyses of the crude product.c Determined by chiral-phase HPLC analyses. | ||||||||
| 1 | L-val–L-phe | 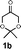 | 4-NO2C6H4 |  | H2O–DMSO, 1 : 1 | 51 | 15 : 1 | 99 |
| 2 | L-val–L-phe | 1b | 4-NO2C6H4 | 2b | H2O–MeOH, 1 : 1 | 83 | 2 : 1 | 99 |
| 3 | L-val–L-val | 1b | 4-NO2C6H4 | 2b | H2O–DMSO, 1 : 1 | 52 | 1 : 1 | 99 |
| 4 | L-val–L-phe | 1a | 4-ClC6H4 |  | H2O–MeOH, 1 : 1 | 65 | 1 : 1 | 86 |
| 5 | L-val–L-phe | 1a | 4-BrC6H4 | 2d | H2O–MeOH, 1 : 1 | 66 | 1 : 1 | 63 |
| 6 | L-val–L-phe |  | 4-NO2C6H4 | 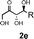 | H2O–DMSO, 1 : 1 | 53 | 1 : 1 | 70 |
| 7 | L-val–L-val | 1c | 4-NO2C6H4 | 2e | H2O–DMSO, 1 : 1 | 67 | 1 : 1 | 51 |
To our delight, the simple linear peptides catalyzed the asymmetric aldol reactions with DHAP mimetic 1b and ketones 1 as the donors with high enantioselectivity and furnished the corresponding aldol products 2b–e in 51–83% yield with up to 99% ee. Notably, the small peptides catalyzed the first enantioselective aldol reactions with dihydroxy acetone (DHA) as the donor. For instance, L-val–L-phe catalyzed the asymmetric assembly of aldol product 2e in 53% yield with 1 : 1 dr and 70% ee (entry 6). In comparison, proline and valine catalyzed the formation of trace amounts of 2e (<10% ee). Thus, small peptides should be considered for the development of direct asymmetric aldol reactions with DHA as the donor.
Our recent density functional theory (DFT) calculations have shown that the primary amino acids utilize a carboxylic acid-catalyzed enamine mechanism (Fig. 1, I).14
 | ||
| Fig. 1 Plausible transition states I and II for the primary amino acid–dipeptide-catalyzed asymmetric aldol reactions. | ||
The generation of nearly racemic aldol products 2 by amino acid catalysis in water indicates that the H2O molecules prevent efficient stabilization of the six-membered transition state by the chiral enamine, which lowers the energy difference between the possible transition states. In contrast, the high enantioselectivity of the small peptide-catalyzed aldol reactions in water suggests that the peptide bond of the natural catalyst is crucial in preserving a stabilized transition state structure, were the Re-face of the chiral enamine is approached by the Si-face of the acceptor aldehyde (Fig. 1, II). This is plausibly accomplished by stabilization of the generated alkoxide of the product by hydrogen bonding by the peptide backbone and the formation of a charge relay system that increases the Brønstedt acidity of the di- and tri-peptides. In fact, a charge relay system is very important in the enamine catalysis of aldolase enzymes.15
In summary, we present that small peptides with a catalytic primary amino acid residue at the N-terminus catalyze the asymmetric intermolecular aldol reaction between unmodified ketones and aldehydes with high stereoselectivity in water. For example, simple di- and tripeptides catalyzed the asymmetric assembly of the corresponding β-hydroxy ketones in up to 86% ee in water and 99% ee in aqueous media. The high modularity of the small peptides should enable the construction of several novel catalysts by combinatorial techniques for the aqueous asymmetric aldol reaction. The remarkably high difference in stereoselectivity between the peptide and amino acid-catalyzed aqueous aldol reactions suggest that peptide bond-formation was an important step towards the evolution of asymmetric catalysis and homochirality. Moreover, the small peptide-catalyzed aqueous aldol reaction may be of biological significance.16,17 Further development of environmentally benign asymmetric C–C bond-forming reactions, mechanistic studies and DFT calculations are ongoing.
We gratefully acknowledge the Swedish National Research Council, Carl-Trygger, Lars-Hierta and Wenner-Gren Foundations for financial support.
References
- (a) W.-D. Fessner, in Stereoselective Biocatalysis, ed. R. N. Patel, Marcel Dekker, New York, 2000, p. 239 Search PubMed; (b) T. D. Machajewski and H. Wong, C.-H., Angew. Chem., Int. Ed., 2000, 39, 1352 CrossRef CAS.
- (a) Comprehensive Organic Synthesis, vol 2., ed. B. M. Trost, I. Fleming and C.-H. Heathcock, Pergamon, Oxford, 1991 Search PubMed; (b) T. Mukaiyama, Angew. Chem., Int. Ed., 2004, 43, 5590 CrossRef CAS.
- Modern Aldol Reactions, vol. 1 & 2, ed. R. Mahrwald, Wiley-VCH, Weinheim, 2004 Search PubMed; E. M. Carreira, in Comprehensive Asymmetric Catalysis, ed. E. N. Jacobsen, A. Pfaltz and H. Yamamoto, Springer, Heidelberg, 1999 Search PubMed; J. S. Johnson and D. A. Evans, Acc. Chem. Res., 2000, 33, 325 Search PubMed; C. Palomo, M. Oiarbide and J. M. García, Chem. Soc. Rev., 2004, 33, 65 Search PubMed . For examples of direct catalytic asymmetric aldol reactions see: K. Juhl, N. Gathergood and K. A. Jørgensen, Chem. Commun., 2000, 2211 CrossRef CAS; B. M. Trost, H. Ito and E. R. Silcoff, J. Am. Chem. Soc., 2001, 123, 3367 RSC; Y. M. A. Yamada, N. Yoshikawa, H. Sasai and M. Shibasaki, Angew. Chem., Int. Ed., 1997, 36, 1871 Search PubMed; N. Yoshikawa, Y. M. A. Yamada, J. Das, H. Sasai and M. Shibasaki, J. Am. Chem. Soc., 1999, 121, 4168 RSC; D. A. Evans, C. W. Downey and J. L. Hubbs, J. Am. Chem. Soc., 2003, 125, 8706 CrossRef CAS.
- For a reviews on asymmetric organic reactions in water, see: U. M. Linström, Chem. Rev., 2002, 102, 2751 Search PubMed; S. Kobayashi and K. Manabe, Chem. Eur. J., 2002, 8, 4094 CrossRef.
- S. Kobayashi and K. Manabe, Acc. Chem. Res., 2002, 35, 209 CrossRef CAS; Y. Mori, K. Manabe and S. Kobayashi, Angew. Chem., Int. Ed., 2001, 40, 2815 CrossRef CAS; C.-J. Li and T.-H. Chan, Tetrahedron, 1999, 55, 11149 CrossRef CAS; A. Graven, M. Groti and M. Meldal, J. Chem. Soc., Perkin Trans. 1, 2000, 955 RSC.
- R. Schoevaart, F. Van Rantwijk and R. A. Sheldon, R. A., J. Org. Chem., 2000, 65, 6940 CrossRef CAS; H. J. M. Gijsen, L. Qiao, W. Fitz and C.-H Wong, Chem. Rev., 1996, 96, 443 CrossRef CAS.
- P. G. Schultz and R. A. Lerner, Nature, 2002, 418, 485 CrossRef CAS.
- For reviews, see: P. I. Dalko and L. Moisan, Angew. Chem., Int. Ed., 2004, 43, 5138 Search PubMed; B. List, Tetrahedron, 2002, 58, 5573 CrossRef CAS . For reviews on the use of peptides in asymmetric organic reactions, see: S. J. Miler, Acc. Chem. Res., 2004, 37, 601 CrossRef CAS; A. Berkessel, Curr. Opin. Chem. Biol., 2003, 7, 409 Search PubMed.
- (a) Z. G. Hajos and D. R. Parrish, J. Org. Chem., 1974, 39, 1615 CrossRef CAS; (b) U. Eder, R. Sauer and R. Wiechert, Angew. Chem., Int. Ed., 1971, 10, 496 CrossRef; (c) B. List, R. A. Lerner and C. F. Barbas, III, J. Am. Chem. Soc., 2000, 122, 2395 CrossRef CAS; (d) W. Notz and B. List, J. Am. Chem. Soc., 2000, 122, 7386 CrossRef CAS; (e) K. S. Sakthivel, W. Notz, T. Bui and C. F. Barbas, III, J. Am. Chem. Soc., 2001, 123, 5260 CrossRef CAS; (f) B. List, P. Porjarliev and C. Castello, Org. Lett., 2001, 3, 573 CrossRef CAS; (g) A. Córdova, W. Notz and C. F. Barbas, III, J. Org. Chem., 2002, 67, 301 CrossRef CAS; (h) A. B. Northrup and D. W. C. MacMillan, J. Am. Chem. Soc., 2002, 124, 6798 CrossRef CAS; (i) A. Bøgevig, N. Kumaragurubaran and K. A. Jørgensen, Chem. Commun., 2002, 620 RSC; (j) Z. Tang, F. Jiang, L.-T. Yu, X. Cui, L.-Z. Gong, A.-Q. Mi, Y.-Z. Jiang and Y.-D. Wu, J. Am. Chem. Soc., 2003, 125, 5262 CrossRef CAS; (k) J. Casas, H. Sundén and A. Córdova, Tetrahedron Lett., 2004, 45, 7697 CrossRef; (l) Torii, M. Nakadai, K. Ishihara, S. Saito and H. Yamamoto, Angew. Chem., Int. Ed., 2004, 43, 1983 CrossRef CAS; (m) A. Hartikaa and P. I. Arvidsson, Tetrahedron: Asymmetry, 2004, 15, 1831 CrossRef CAS; (n) A. Berkessel, B. Koch and J. Lex, Adv. Synth. Catal., 2004, 346, 1141 CrossRef CAS; (o) A. J. A. Cobb, D. M. Shaw, D. A. Longbottom, J. B. Gold and S. V. Ley, Org. Biomol. Chem., 2005, 3, 84 RSC; (p) J. Casas, M. Engqvist, I. Ibrahem, B. Kaynak and A. Córdova, Angew. Chem., Int. Ed., 2005, 44, 1343 CrossRef; (q) D. Enders and C. Grondal, Angew. Chem., Int. Ed., 2005, 44, 1210 CrossRef CAS; (r) I. Ibrahem and A. Córdova, Tetrahedron Lett., 2005, 46, 3363 CrossRef CAS; (s) A. I. Nyberg, A. Usano and P. Pihko, SYNLETT, 2004, 1891 CAS; (t) D. E. Ward and V. Jheengut, Tetrahedron Lett., 2004, 45, 8347 CrossRef CAS. For examples of N-terminal prolyl peptide-catalyzed asymmetric aldol reactions, see: (u) J. Kofoed, J. Nielsen and J.-L. Reymend, Bioorg. Med. Chem. Lett., 2003, 13, 2445 CrossRef CAS; (v) H. J. Martin and B. List, SYNLETT, 2003, 1901 CAS; (w) Z. Tang, Z.-H. Yang, L.-F. Cun, L.-Z. Gong, A.-Q. Mi and Y.-Z. Jiang, Org. Lett., 2004, 6, 2285 CrossRef CAS; (x) P. Krattiger, R. Kovasy, J. D. Revell, S. Ivan and H. Wennemers, Org. Lett., 2005, 7, 119 CrossRef.
- J. L. Reymond and Y. Chen, J. Org. Chem., 1995, 60, 6970 CrossRef CAS; T. J. Dickerson and K. D. Janda, J. Am. Chem. Soc., 2000, 122, 7386 CrossRef CAS; A. Córdova, W. Notz and C. F. Barbas, III, Chem. Commun., 2002, 3024 RSC; S. S. Chimni, D. Mahajan and V. V. S. Babu, Tetrahedron Lett., 2005, 46, 5617 CrossRef CAS; Y.-Y. Peng, Q.-P. Ding, Z. Li, P. G. Wang and J.-P. Cheng, Tetrahedron Lett., 2003, 44, 3871 CrossRef CAS . For examples of aqueous aldol reactions promoted by amino acid Zn complexes, see: M. Nakagawa, H. Nakao and K.-I. Watanabe, Chem. Lett., 1985, 391 Search PubMed; T. Darbre and M. Machuqueiro, Chem. Commun., 2003, 1090 CAS; J. Kofoed, M. Machuqueiro, J.-L. Reymond and T. Darbre, Chem. Commun., 2004, 1540 RSC.
- S. Pizzarello and A. Weber, Science, 2004, 303, 1151 CrossRef CAS; A. Córdova, M. Engqvist, I. Ibrahem, J. Casas and H. Sundén, Chem. Commun., 2005, 2047 RSC; A. Córdova, I. Ibrahem, J. Casas, H. Sundén, M. Engqvist and E. Reyes, Chem. Eur. J., 2005, 11, 4772.
- (a) A. Córdova, W. Zou, I. Ibrahem, E. Reyes, M. Engqvist and W.-W. Liao, Chem. Commun., 2005, 3586 RSC; (b) W. You, I. Ibrahem, P. Dziedzic, H. Sundén and A. Córdova, Chem. Commun., 2005 10.1039/b509902j.
- R. Breslow, Chem. Rev., 1998, 98, 1997 CrossRef CAS; D. Q. Yuan, S. D. Dong and R. Breslow, Tetrahedron Lett., 1998, 39, 7673 CrossRef CAS; R. Breslow and A. Graff, J. Am. Chem. Soc., 1993, 115, 10988 CrossRef CAS.
- A. Bassan, W. Zou, E. Reyes, F. Himo and A. Córdova, Angew. Chem., Int. Ed., 2005, 44, 7028 CrossRef.
- A. Heine, G. Desantis, J. G. Luz, M. Mitchell and C.-H. Wong, Science, 2001, 294, 369 CrossRef CAS.
- We found that small peptides isolated from the cytosol of human melanoma cells catalyzed the formation of 2a in 54% yield with 75% ee. In addition, bovine serum albumin mediated the asymmetric formation of 2a with 9% ee in water.
- Small peptides (<40 amino acids) catalyze aldol reactions and related reactions in buffer. However, the reactions were not enantioselective: H. D. Dakin, J. Biol. Chem., 1910, 7, 49 Search PubMed; K. Johnsson, R. K. Allemann, H. Widmer and S. A. Benner, Nature, 1993, 365, 530 CAS; C. J. Weston, C. H. Cureton, M. J. Calvert, O. S. Smart and R. K. Allemann, ChemBioChem, 2004, 5, 1075 CrossRef; F. Tanaka, R. Fuller and C. F. Barbas, III, Biochemistry, 2005, 44, 7583 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, NMR data and amino acid analyses. See DOI: 10.1039/b515880j |
| This journal is © The Royal Society of Chemistry 2006 |